ヒドリドカリウム 化学特性,用途語,生産方法
化学的特性
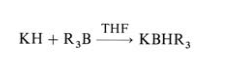
Potassium hydride is available in laboratory quantities only as a 20 – 35 % dispersion in oil. Potassium hydride is a considerably stronger base than lithium hydride or sodium hydride. It is able to remove protons from tertiary alcohols and ketones, a reaction that either does not occur or is very slow when sodium hydride is used. Potassium hydride also reacts with weak Lewis acids, converting sterically hindered boron trialkyls to the corresponding sterically hindered complex borohydrides:
使用
Potassium Hydride is used in preparation of Hydroxy-Xanthone derivatives via Isoprenylation followed by Claisen rearrangement starting from Fluoroxanthone derivatives.
定義
potassium hydride: A white orgreyish white crystalline solid, KH;r.d. 1.43–1.47. It is prepared by passinghydrogen over heated potassiumand marketed as a light grey powderdispersed in oil. The solid decomposeson heating and in contact withmoisture and is an excellent reducingagent. Potassium hydride is a firehazard because it produces hydrogenon reaction with water.
反応性
Potassium hydride acts as a base and as hydride donor. It is used for deprotonation, cyclization-condensation, elimination, and rearrangement reactions, and also as a reducing agent. Potassium hydride undergoes reaction quickly and quantitatively with acids, and of particular note is its capability to rapidly deprotonate tertiary alcohols where sodium hydride or potassium metal do so slowly or not at all. The reactions of metal hydrides take place at the crystal surface. The crystal lattice energies decrease from lithium to cesium hydride, and potassium hydride appears to have the optimal lattice energy and hydride radius for surface reactions. The presence of 18-crown-6 enhances the reactivity of potassium hydride, The crown ether can operate as a phase-transfer agent or as a simple “pickling” agent of the potassium hydride surface, dissolving the formed inorganic salts. Potassium hydride is usually superior to lithium and sodium hydride in the reactions. Unusually active potassium hydride can be prepared easily from hydrogen and superbasic reagents (t-BuOK-TMEDA) in hexane. “Superactive potassium hydride” is very active in deprotonation as well as in reduction. The reactivity of commercially available potassium hydride, which is prepared by the reaction of hydrogen gas with elemental potassium, depends upon the impurities in different lots (mainly potassium or its reaction products), thus leading to side reactions and variable yields. The superactive metal hydride contains no alkali metal.
一般的な説明
This product has been enhanced for energy efficiency.
危険性
Dangerous fire and explosion risk, evolves
toxic and flammable gases on heating and on expo-
sure to moisture.
健康ハザード
Potassium hydride react with the moisture on skin and other
tissues to form highly corrosive sodium and potassium hydroxide. Contact of these
hydrides with the skin, eyes, or mucous membranes causes severe burns; thermal
burns may also occur due to ignition of the liberated hydrogen gas.
火災危険
Potassium hydride is flammable solid that ignite on contact
with moist air. Potassium hydride presents a more serious fire hazard than sodium
hydride. The mineral oil dispersions do not ignite spontaneously on exposure to the
atmosphere. Sodium hydride and potassium hydride fires must be extinguished with
a class D dry chemical extinguisher or by the use of sand, ground limestone, dry clay
or graphite, or "Met-L-X ? " type solids. Water or CO 2 extinguishers must never be
used on sodium and potassium hydride fires.
燃焼性と爆発性
Potassium hydride and sodium hydride are flammable solids that ignite on contact
with moist air. Potassium hydride presents a more serious fire hazard than sodium
hydride. The mineral oil dispersions do not ignite spontaneously on exposure to the
atmosphere. Sodium hydride and potassium hydride fires must be extinguished with
a class D dry chemical extinguisher or by the use of sand, ground limestone, dry clay
or graphite, or "Met-L-X?" type solids. Water or CO2 extinguishers must never be
used on sodium and potassium hydride fires.
安全性プロファイル
Dangerous fire hazard
by chemical reaction. Ignites spontaneously
in air. Moderate explosion hazard when
exposed to heat or by chemical reaction.
Wdl react with water, steam, or acids to
produce H2 which then igmtes. Can react
vigorously with oxidizing materials. To fight
fire, use CO2, dry chemical. Potentially
explosive reactions with 0-2,4-
dnitrophenylhydroxylamine, fluoroalkenes.
Ignites on contact with air, oxygen +
moisture, fluorine. Incompatible with Cl2,
acetic acid, acrolein, acrylonitrile, (CaC +
Cl2), ClO2, (H202 + Cl2), (CHFL +
CH,OH), 1,2-dchloroethylene, maleic
anhydride, (n-methyl-n-nitrosourea +
CH2Cl2), nitroethane, NCb, nitromethane,
nitroparaffins, o-nitrophenol, nitropropane,
n-nitrosomethylurea, (nitrosomethylurea +
CH2Cl2), H20, trichloroethylene,
tetrahydrofuran, tetrachlorethane. When
heated to decomposition it emits highly
toxic fumes of K2O. See also POTASSIUM
and HYDRIDES.
貯蔵
Safety glasses, impermeable gloves, and a fire-retardant laboratory
coat should be worn at all times when working with these substances. These
hydrides should be used only in areas free of ignition sources and should be stored
preferably as mineral oil dispersions under an inert gas such as argon.
不和合性
Potassium hydride and sodium hydride react violently with water, liberating
hydrogen, which can ignite. Oil dispersions of these hydrides are much safer to
handle because the mineral oil serves as a barrier to moisture and air. Potassium
hydride may react violently with oxygen, CO, dimethyl sulfoxide, alcohols, and
acids. Explosions can result from contact of these compounds with strong oxidizers.
Potassium hydride is generally more reactive than sodium hydride.
廃棄物の処理
Excess potassium or sodium hydride and waste material containing these substances should be placed in an
appropriate container under an inert atmosphere, clearly labeled, and handled according to your institution's
waste disposal guidelines. Experienced personnel can destroy small quantities of sodium hydride and
potassium hydride by the careful dropwise addition of t-butanol or iso-propanol to a suspension of the
metal hydride in an inert solvent such as toluene under an inert atmosphere such as argon. Great care must
be taken in the destruction of potassium hydride because of its greater reactivity. The resulting mixture of
metal alkoxide should be placed in an appropriate container, clearly labeled, and handled according to your
institution's waste disposal guidelines.
ヒドリドカリウム 上流と下流の製品情報
原材料
準備製品