アゼライン酸 化学特性,用途語,生産方法
外観
白色~わずかにうすい黄色, フレーク又は粉末
定義
本品は、次の化学式で表されるジカルボン酸である。
溶解性
エタノール及びアセトンに溶け、水に溶けにくい。
用途
合成繊維、合成樹脂、可塑剤原料
化粧品の成分用途
pH調整剤、香料
効能
抗ニキビ薬, チオレドキシン還元酵素阻害薬
説明
Azelaic acid is a topical antiacne agent which exerts its therapeutic action through a
myriad of antimicrobial, antiproliferative and cytostatic effects. In vitro, azelaic acid hasbeen shown to inhibit DNA polymerases in several tumor cell lines.
化学的特性
Azelaic acid is an organic compound with the formula (CH
2)
7(CO
2H)
2. This saturated dicarboxylic acid exists as a white powder. It is found in wheat, rye, and barley. It is a component of a number of hair and skin conditioners.
使用
Azelaic acid is used in lacquers, alkyd resins, plasticizers,
adhesives, polyamides, urethane elastomers, and organic
syntheses. Azelaic acid is also used in treating of
acne.
調製方法
Azelaic acid is industrially produced by the ozonolysis of oleic acid. The side product is nonanoic acid. It is produced naturally by Malassezia furfur (also known as Pityrosporum ovale), a yeast that lives on normal skin. The bacterial degradation of nonanoic acid gives azelaic acid.
適応症
Azelaic acid (Azelex) is a naturally occurring dicarboxylic
acid produced by the yeast Malassezia furfur.
Azelaic acid inhibits tyrosinase, a rate-limiting enzyme
in the synthesis of the pigment melanin. This may explain
why diminution of melanin pigmentation occurs in
the skin of some patients with pityriasis versicolor, a disease
caused by M. furfur. Azelaic acid is bacteriostatic
against a number of species thought to participate in the
pathogenesis of acne, including Propionibacterium acnes.
The drug may also reduce microcomedo formation
by promoting normalization of epidermal keratinocytes.
製造方法
Azelaic acid is made by the ozonolysis of oleic acid:
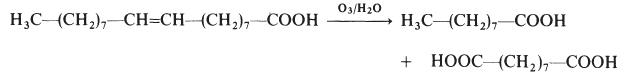
主な応用
Azelaic acid, also known as azalea acid, is a white to slightly yellow powder. Azelaic acid is a medium-long chain dibasic acid[1]. In recent years, with the rapid development of the organic synthetic chemical industry, the demand for medium and long chain dibasic acids is increasing. The medium and long chain dibasic acids and their derivatives have a wide range of industrial applications and a broad product market.
一般的な説明
Azelaic acid is used as a therapeutic agent in dermatology.
生物学的応用
In plants, azelaic acid serves as a "distress flare" involved in defense responses after infection. It serves as a signal that induces the accumulation of salicylic acid, an important component of a plant's defensive response.
作用機序
Naturally occurring dicarboxylic acid that is bacteriostatic
to Propionibacterium acnes. It also decreases conversion of testosterone
to 5{pi}ga-dihydrotestosterone (DHT) and alters keratinization of the microcomedone.
It may also be beneficial in the treatment of melasma. The
mechanism of action is not fully understood. Deoxyribonucleic acid (DNA)
synthesis is reduced, and mitochondrial cellular energy products are inhibited
in melanocytes.
臨床応用
Azelaic acid is used for the treatment of mild to
moderate acne, particularly in cases characterized by
marked inflammation-associated hyperpigmentation.
安全性プロファイル
Low toxicity by ingestion. A skinand eye irritant. Closely related to glutaric acid and adipicacid. Combustible when exposed to heat or flame; canreact with oxidizing materials.
純化方法
Recrystallise it from H2O(charcoal) or thiophene-free *benzene. The acid can be dried by azeotropic distillation with toluene, the residual toluene solution is then cooled and filtered, and the precipitate is dried in a vacuum oven. It has been purified by zone refining or by sublimation onto a cold finger at 10-3torr. It distils above 360o with partial formation of the anhydride. The dimethyl ester has m –3.9o and b 140o/8mm. [Hill & McEwen Org Synth Coll Vol II 53 1943, Beilstein 2 IV 2055.]
アゼライン酸 上流と下流の製品情報
原材料
準備製品