ペルオキシバリウム 化学特性,用途語,生産方法
解説
過酸化バリウム,BaO2(169.33).水酸化バリウムの飽和水溶液に過酸化水素水を加えると,八水和物BaO2・8H2Oが得られる.また,BaO粉末を乾燥した酸素気流中500 ℃ で加熱すると無水物が得られる.無水物は白色の粉末.密度4.96 g cm-3.融点450 ℃.840 ℃ でBaOと O2 とに分解する.水に微溶.熱水では分解してBa(OH)2と O2 になる.酸を作用させると,H2O2を生じて分解する.八水和物は白色で光沢のある結晶.密度2.29 g cm-3.空気中におくとCO2の作用で徐々に炭酸塩になる.有機物に触れると発火する.低温で希硫酸を加えるとH2O2水溶液を生じるので,かつてはH2O2の製造原料として用いられていた.酸化剤,漂白剤,X線防御用ガラスの製造などに用いられる.有毒.八水和物は無色、六方晶系の結晶で、100℃で無水物に変わる。無水物よりはいくぶん水に溶けやすい。酸化剤として織物、そばの漂白、過酸化水素の製造原料のほか、爆発時を調整する延時薬として火薬の製造に使われる。
用途
酸化剤,漂白剤,火薬原料 (NITE CHRIP)
性質
化学式BaO2。酸化バリウムBaOを空気または酸素中で500℃に加熱すると得られる。また,水酸化バリウム水溶液に過酸化水素を加えると8水和物BaO2・8H2Oが沈殿する。無水和物は無色,正方晶系の粉末で,比重4.95。バリウムイオンBa2+と過酸化物イオン
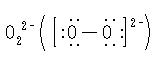
とから成るイオン結晶で,カルシウムイオンCa2+とアセチレン化物イオン
とから成るカルシウムカーバイドと同様の構造をもつ。高温では分解して酸素を放つ。
説明
Barium peroxide, is a grayish-white powder that is slightly soluble in water. Barium peroxide is a dangerous fire and explosion risk in contact with organic materials and decomposes around 1450°F (787°C). It is also toxic by ingestion, is a skin irritant, and should be kept cool and dry in storage. The four-digit UN identification number is 1449. The primary uses of barium peroxide are in bleaching, in thermal welding of aluminum, as an oxidizing agent, and in the dyeing of textiles.
化学的特性
Barium peroxide is a grayish-white powder.
物理的性質
BaO2 is an iron gray or white powder. It is slowly
decomposed in air, forming the hydroxide and oxygen.
It does not dissolve in water, but can slowly hydrolyze,
forming hydrogen peroxide in solution. Barium peroxide is a strong oxidizing agent and will explode if direct contact with organic matter occurs. Therefore, barium peroxide is always diluted to form a slurry before usage.
Barium peroxide is a strong oxidizing agent that is
used for bleaching. Barium peroxide contains O2
2-
subunits wherein the oxygen atoms bond to each other
as well as to the barium.
使用
Bariumperoxide is used as a hydrogen peroxide source
and oxygen oxidant, as well as a bleaching agent. Its main
usage has been for making hydrogen peroxide and
oxygen, in organic syntheses, fabric printing and dyeing.
Barium peroxide is available commercially, primarily
as the oxctahydrate (which is the more stable form of
this peroxide).
定義
barium peroxide: A dense offwhitesolid, BaO
2, prepared by carefullyheating barium oxide inoxygen; r.d. 4.96; m.p. 450°C. It is used as a bleaching agent. Withacids, hydrogen peroxide is formedand the reaction is used in the laboratorypreparation of hydrogen peroxide.
製造方法
Barium peroxide is best prepared by reacting barium
nitrate with sodium peroxide in a cold solution:
Ba(NO3)2+Na2O2+xH2O→BaO2·xH2O+2NaNO3
The hydrated form is usually the octahydrate. If the
anhydrate is desired, the hydrated peroxide is dried
and then sintered at 350°C for 10 min or less:
4BaO2·xH2O+ heat→2BaO+2BaO2+xH2O+O2
About equal amounts of oxide and peroxide form.
The ratio is a function of the time and temperature of
heating. To separate the two forms, the heated mass is
plunged into a large volume of water where the
hydroxide is formed. The peroxide is insoluble whereas
the hydroxide is soluble, allowing the separation of the
two by filtration. The peroxide is then vacuum dried.
調製方法
Barium peroxide, BaO2, was the first-known peroxo compound. It was used until mid-1900 in the manufacture of oxygen by the Brin process and of hydrogen peroxide by the Thenard reaction.
一般的な説明
A grayish-white granular solid. Insoluble in water. Noncombustible, but accelerates the burning of combustible material. Mixture with finely divided combustible material may be explosive. Mixtures with combustible material may be ignited by friction or contact with moisture.
空気と水の反応
Decomposed by water. Insoluble in water.
反応プロフィール
Barium peroxide is a strong oxidizing agent. Contact with water can produce a temperature and oxygen concentration high enough to ignite organic materials [Bretherick's, 5th ed., 1995, p. 94]. Reacts explosively with acetic anhydride due to the formation of acetyl peroxide [Rust, 1948, p. 337]. Ignites when mixed with powdered aluminum, powdered magnesium or calcium-silicon alloys. Wood may ignite with friction from the peroxide. Decomposes when heated to 700°C to produce barium oxide and pure oxygen [Sax, 9th ed., 1996, p. 317]. Forms highly reactive mixtures with fuel-type materials.
危険性
Oxidizing material. Fire and explosion risk
in contact with organic materials. Keep cool and
dry. Toxic by ingestion, skin irritant.
健康ハザード
Inhalation causes irritation of mucous membranes, throat, and nose. Contact with eyes or skin causes severe burns. Ingestion causes excessive salivation, vomiting, colic, diarrhea, convulsive tremors, slow, hard pulse, and elevated blood pressure; hemorrhages may occur in the stomach, intestines, and kidneys; muscular paralysis may follow.
火災危険
Behavior in Fire: Can increase intensity of fire.
安全性プロファイル
A poison via
subcutaneous route. A powerful oxidtzer.
Explodes on contact with acetic anhydride.
Ignites when mixed with calcium-silicon
alloys, powdered aluminum, powdered
magnesium, water + organic compounds.
Mixtures with propane react violently when
heated. The powder ignites when heated to
265℃ with selenium. Wood ignites with
friction from the peroxide. Incompatible
with H2S, water, peroxyformic acid,
hydroxylamine solution, mixture of (Mg +
Zn + Ba(NO3)2), and organic matter. See
also BARIUM COMPOUNDS (soluble)
and PEROXIDES, INORGANIC.
職業ばく露
Is used as a bleaching agent; in making
hydrogen peroxide, oxygen; in aluminum welding; in
textile dyeing and for bleaching fibers; animal substances.
輸送方法
UN1449 Barium peroxide, Hazard Class: 5.1;
Labels: 5.1—Oxidizer, 6.1—Poisonous materials.
不和合性
A strong oxidizer. Keep away from
organic and combustible materials (such as wood, paper,
oil, fuels, and other easily oxidized materials) and peroxyformic
acid, hydrogen sulfide and hydroxylamine solutions,
since violent reactions occur.
廃棄物の処理
Dispose of contents and container
to an approved waste disposal plant. All federal,
state, and local environmental regulations must be
observed. Contact your local or federal environmental protection
agency for specific recommendations.
ペルオキシバリウム 上流と下流の製品情報
原材料
準備製品