シクロペンタン 化学特性,用途語,生産方法
外観
無色~ほとんど無色, 澄明の液体
準備
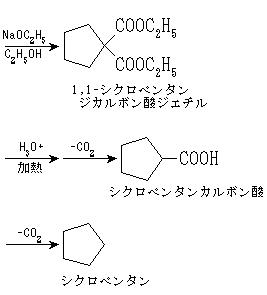
5員環の炭化水素。シクロペンタ,常温で無色の可燃性液体。融点-93.46℃,沸点49.26℃。1,4‐ジブロモブタンとマロン酸ジエチルとをナトリウムエトキシドの存在下に反応させて得られる1,1‐シクロペンタンジカルボン酸ジエチルを,加水分解,脱炭酸してシクロペンタンカルボン酸とし,さらにこれを脱炭酸して合成する。
最も安定な配座は,下図のように4個の炭素原子が同一平面上に並び,残りの1個の炭素原子がその面の外に位置した〈封筒形〉と呼ばれる配座である。
性質
1. シクロペンタンの構造
シクロペンタンはシクロアルカンまたは脂環式炭化水素と呼ばれる環状構造の炭素骨格をもつ環状飽和炭化水素の1つです。ここで、飽和というのは炭素原子間の結合がすべて飽和結合 (単結合) であることを表しています。
シクロアルカンの一般式は(CH2)n または CnH2nであり、骨格構造は多角形で表されます。シクロペンタンの骨格構造は五角形です。
2. シクロペンタンの安定性
シクロアルカンの炭素数は3以上ですが、炭素数3のシクロプロパンや炭素数4のシクロブタンは化学的に不安定で、環状構造が開いて鎖状のアルカンに変化しやすいという性質を持っています。これは炭素結合の結合角のひずみに起因するものです。
典型的な炭素の結合角は109°に対して、シクロプロパン (正三角形) の結合角は60°、シクロブタン (正方形) の結合角は90°と大きくずれています。このような角ひずみは不安定さの要素であり、簡単に結合が切れる原因となります。
一方、シクロペンタン (正五角形) の結合角は108°でありほとんど角ひずみがありません。水素の数が多いためねじれひずみがあり、折れ曲がった立体配座になっていますが、化学的には安定しています。
溶解性
水に不溶, エタノール, エーテルに混和。エタノール及びアセトンに極めて溶けやすく、水にほとんど溶けない。
解説
C5H10(70.13).ガソリンの精密分留によって得られるが,ペンタンの高温脱水素環化によっても合成される.
"無色の液体.融点-94 ℃,沸点49.3 ℃.シクロパラフィン中ではもっとも安定である.[CAS 287-92-3]
森北出版「化学辞典(第2版)
用途
有機合成原料、溶剤。
用途
セルロースエーテル用溶剤、自動車燃料、共沸蒸留用
化学的特性
Cyclopentane is a colourless flammable acyclic hydrocarbon liquid with a petrol-like smell. It is a widely used component in preparing products like analgesics, insecticides, sedatives, antitumor agents and also finds application in pharmaceutical industry.
物理的性質
Colorless, mobile, flammable liquid with an odor resembling cyclohexane.
使用
Cyclopentane is a petroleum product. Itis formed from high-temperature catalyticcracking of cyclohexane or by reduction ofcyclopentadiene. It occurs in petroleum etherfractions and in many commercial solvents.It is used as a solvent for paint, in extractionsof wax and fat, and in the shoe industry.
調製方法
Cyclopentane occurs in petroleum ether fractions and is
prepared by cracking cyclohexane in the presence of alumina
at high temperature and pressure or by reduction of cyclopentadiene.
定義
ChEBI: A cycloalkane that consists of five carbons each bonded with two hydrogens above and below the plane. The parent of the class of cyclopentanes.
一般的な説明
A clear colorless liquid with a petroleum-like odor. Flash point of -35°F. Less dense than water and insoluble in water. Vapors are heavier than air.
空気と水の反応
Highly flammable. Insoluble in water.
反応プロフィール
CYCLOPENTANE is incompatible with strong oxidizing agents such as chlorine, bromine, fluorine. .
健康ハザード
Cyclopentane is a low-acute toxicant. Itsexposure at high concentrations may producedepression of the central nervous system withsymptoms of excitability, loss of equilibrium,stupor, and coma. Respiratory failure may occur in rats from 30–60 minutes’ exposureto 100,000–120,000 ppm in air. It is anirritant to the upper respiratory tract, skin,and eyes. No information is available inthe literature on the chronic effects fromprolonged exposure to cyclopentane.
火災危険
Behavior in Fire: Containers may explode.
ペンタンとの比較
環状のシクロアルカンに対して、鎖状の飽和炭化水素がアルカンです。シクロアルカンの物理・化学的性質は鎖状アルカンと非常によく似ています。
炭素5つの鎖状アルカンはペンタンです。ペンタンもシクロペンタンも常温で液体で可燃性 (引火性) があります。また、両者とも光を当ててを加えれば置換反応を起こします。
使用用途
1. 発泡剤
シクロペンタンは、セルロースエーテル用の溶剤や住宅のなどに使用されているといった樹脂の発泡剤に使われています。
2. ハイオクガソリン
また、シクロペンタンは、ガソリンへ添加することで、自動車からの排気ガスに含まれる有害物質を減少させることや自動車のエンジン効率に関わりのあるオクタン価向上にも寄与するという実験結果が公表されています。
オクタン価とは、エンジンノックの起こりにくさの程度を示す目安です。古くから鎖状アルカンは高次に分岐した化合物よりもエンジンノックを起こしやすいことが知られていました。環状アルカンであるシクロペンタンは同じ炭素数のよりオクタン価が高くなります。
3. 共沸蒸留の溶媒
そのほか、シクロペンタンは、共沸 (きょうふつ) 混合物の分離手段としての蒸溜にも使用されています。共沸とは沸点が異なる2つの液体の混合物が組成を変えずに沸騰する現象です。
共沸混合物から通常の蒸留法を用いて純粋な物質を取り出すことは困難です。そこで、元の成分とは別の溶媒を加えて新たな共沸混合物をつくり、その沸点が元の沸点よりも低くなるように蒸留し、純粋な成分が残留するようにします。この方法を共沸蒸留と呼びます。
化学反応性
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
安全性プロファイル
Mildly toxic by
ingestion and inhalation. High
concentrations have narcotic action. A very
dangerous fire hazard when exposed to heat
or flame; can react with oxidizers. To fight
fire, use foam, CO2, dry chemical. When
heated to decomposition it emits acrid
smoke and fumes.
職業ばく露
Cyclopentane is used as a solvent.
概要
シクロペンタンとは、化学式:C5H10、分子量:70.13、CAS登録番号:287-92-3の有機化合物です。
シクロペンタンは、無色~ほとんど無色の澄明 (ちょうめい) な特異臭のある液体です。融点/凝固点が-94℃で、沸点または初留点および沸騰範囲が49℃です。そして、水にはほとんど溶けず、・に極めて溶けやすいという性質があります。
環境運命予測
Biological. Cyclopentane may be oxidized by microbes to cyclopentanol, which may oxidize to
cyclopentanone (Dugan, 1972).
Photolytic. The following rate constants were reported for the reaction of octane and OH
radicals in the atmosphere: 3.7 x 10
-12 cm
3/molecule?sec at 300 K (Hendry and Kenley, 1979); 5.40
x 10
-12 cm
3/molecule?sec (Atkinson, 1979); 4.83 x 10
-12 cm
3/molecule?sec at 298 K (DeMore and
Bayes, 1999); 6.20 x 10
-12, 5.24 x 10
-12, and 4.43 x 10
-12 cm
3/molecule?sec at 298, 299, and 300 K,
respectively (Atkinson, 1985), 5.16 x 10
-12 cm
3/molecule?sec at 298 K (Atkinson, 1990), and 5.02
x 10
-12 cm
3/mol·sec at 295 K (Droege and Tilly, 1987).
Chemical/Physical. Cyclopentane will not hydrolyze because it has no hydrolyzable functional
group. Complete combustion in air yields carbon dioxide and water.
At elevated temperatures, rupture of the ring occurs forming ethylene and presumably allene
and hydrogen (Rice and Murphy, 1942).
輸送方法
UN1146 Cyclopentane, Hazard Class: 3; Labels:
3-Flammable liquid.
純化方法
Free it from cyclopentene by two passages through a column of carefully dried and degassed activated silica gel. It occurs in petroleum and is HIGHLY FLAMMABLE. [NMR: Christl Chem Ber 108 2781 1975, Whitesides et al. 41 2882 1976, Beilstein 5 III 10, 5 IV 4.]
不和合性
May form explosive mixture with air.
May accumulate static electrical charges, and may cause
ignition of its vapors. Contact with strong oxidizers may
cause fire and explosion.
廃棄物の処理
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinera-
tor equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be
observed.
シクロペンタン 上流と下流の製品情報
原材料
イソペンタン
ペンタン
シクロペンタノン
シクロペンタジエン
4,7-Methano-1H-indene, 3a,4,5,6,7,7a-hexahydro-, (3aR,4S,7R,7aR)-rel-
(1S,2S)-1,2-シクロペンタンジオール
シクロペンタンメタノール
シクロペンチルシクロペンタン
シクロペンチルマグネシウムブロミド (18%テトラヒドロフラン溶液, 約1mol/L)
シクロペンタン-1α,2α-ジオール
メチルシクロペンタン
フェノール
シクロペンテン
準備製品