ジフェニルアミン 化学特性,用途語,生産方法
外観
白色~わずかにうすい黄色, 結晶~結晶性粉末
種類
ジフェニルアミンは、主に研究開発用試薬製品や工業用化学薬品として販売されている物質です。通常、冷蔵保管が必要な試薬製品です。
工業用化学製品としては、20kg紙袋などの容量の単位で販売されます。用途としては、染料中間体、ニトロセルロースやセルロイドなどの安定が想定されています。
性質
ジフェニルアミンは、分子式 C12H11N、分子量169.23、融点53℃、沸点302℃であり、常温では芳香を持つ白色または黄色の結晶固体です。密度は1.15g/mL、酸解離定数pKaは0.78です。水には不溶ですが、エタノール、アセトン、ベンゼン、四塩化炭素、ピリジン、酢酸エチルに容易に溶けます。
それ以外には、エーテル、酢酸に可溶であり、クロロホルムにわずかに溶けます。
溶解性
エタノール, ジエチルエーテルにやや溶けやすく水にはほとんど溶けない。エタノール及びジエチルエーテルにやや溶けやすく、水にはほとんど溶けない。
解説
芳香族アミンの一つ。ジフェニルアミンは,芳香をもつ無色の結晶。弱塩基性。アニリンから合成される。糖類の呈色反応、硝酸塩やDNAの定量のほか、硝煙反応に用いられる。アニリンの塩酸塩と小過剰のアニリンの混合物を、圧力をかけながら加熱すると得られる無色の結晶。芳香がある。塩基性は弱い。水には溶けないが、エタノール(エチルアルコール)、エーテル、ベンゼンなどによく溶ける。濃硫酸溶液に硝酸イオンや亜硝酸イオンを加えると濃青色を呈するので、これらのイオンの検出剤として使われる(ジフェニルアミン反応)。また核酸の定量試薬としても使われる。アゾ染料の合成中間体、ニトロセルロースやセルロイドの安定剤として用いられる。発癌(はつがん)性が認められている。
解説
C12H11N(169.22).(C6H5)2NH.アニリンと塩酸アニリンとの等モル混合物をオートクレーブ中で加熱すると得られる芳香族第二級アミン.葉状晶.融点54 ℃,沸点302 ℃.密度1.16 g cm-3.アルコール類に易溶.その濃硫酸水溶液は,微量の硝酸または亜硝酸によって青色を呈するので,これらの検出に用いる.工業的には,染料や医薬品の中間物として用いられる.[CAS 122-39-4]
森北出版「化学辞典(第2版)
用途
硝酸塩の検出試薬、有機合成原料(染料、医薬品)、安定剤(火薬、塩素系溶剤)。
用途
有機ゴム薬品、染料、火薬安定剤、塩素系溶剤の安定剤、医薬品
用途
染料中間体、媒染剤
合成
ジフェニルアミンは、とを加圧、加熱することで得られます。また、酸化物触媒存在下におけるアニリンの脱アミノ化反応でも合成可能です。
農薬用途
殺菌剤
使用上の注意
不活性ガス封入光によって徐々に着色する。
説明
Solutions of diphenylamine are used to treat apples a few days
before harvest. Residue on apples’ surfaces of 10 ppm is
permitted by regulation in Australia, Canada, and the United
States.
化学的特性
Diphenylamine is a colorless monoclinic leafl et substance. It is used in the manufacture
of a variety of substances, i.e., dyestuffs and their intermediates, pesticides, antihelmintic
drugs, and as reagents in analytical chemistry laboratories.
使用
Diphenylamine is an aromatic amine that was shown to exhibit antioxidant activities and is now used as an anti-scald agent. It is also used in the manufacture of a variety of substances, for instance, dye stuffs and their intermediates, pesticides, anthelmintic drugs, and as reagents in analytical chemistry laboratories.
定義
diphenylamine: A colourless crystallinearomatic compound,(C
6H
5)
2NH; m.p. 54°C. It is made byheating phenylamine (aniline) withphenylamine hydrochloride. It is asecondary amine and is both slightlyacidic (forming an N-potassium salt)and slightly basic (forming salts withmineral acids). Its derivatives are employedas stabilizers for syntheticrubber and rocket fuels.
一般的な説明
Light tan to brown solid with a pleasant odor. Sinks in water.
空気と水の反応
Dust may be explosive if mixed with air in critical proportions and in the presence of a source of ignition [USCG, 1999]. Insoluble in water.
反応プロフィール
Diphenylamine discolors in light. Diphenylamine can react violently with hexachloromelamine and trichloromelamine. Diphenylamine is incompatible with strong oxidizing agents and strong acids. Diphenylamine is also incompatible with iron and silver salts. Diphenylamine reacts with nitrogen oxides.
健康ハザード
Diphenylamine is highly toxic and is rapidly absorbed by the skin and through inhalation.
It has caused anorexia, hypertension, eczema, and bladder symptoms. Experimental
animals exposed to diphenylamine demonstrated cystic lesions but failed to demonstrate
cancerous growth. Inhalation of diphenylamine dust may cause systemic poisoning. The
symptoms of toxicity include, but are not limited to, anoxia, headache, fatigue, anorexia,
cyanosis, vomiting, diarrhea, emaciation, hypothermia, bladder irritation, kidney, heart,
and liver damage.
火災危険
Noncombustible solid; autoignition temperature 634°C (1173°F); low reactivity.
使用用途
ジフェニルアミンの主な使用用途は、硝酸塩の検出試薬、各種有機合成原料・中間体、火薬・塩素系溶剤の安定剤などです。特に、硝酸塩の検出は硝煙反応として知られています。また、DNAの抽出にも使われる物質です。
合成原料としては、 医薬・染料・有機ゴム薬品や、重合禁止剤であるフェノチアジンなどの合成中間体・原料物質として使用されます。また、有機ゴム薬品の老化防止剤 (N-(1,3)-ジメチルブチル-N'-フェニル-p-フェニレンジアミンなど) の合成原料でもあります。染料では、酸性および硫化系およびセリトン染料に用いられる物質です。
呈色反応
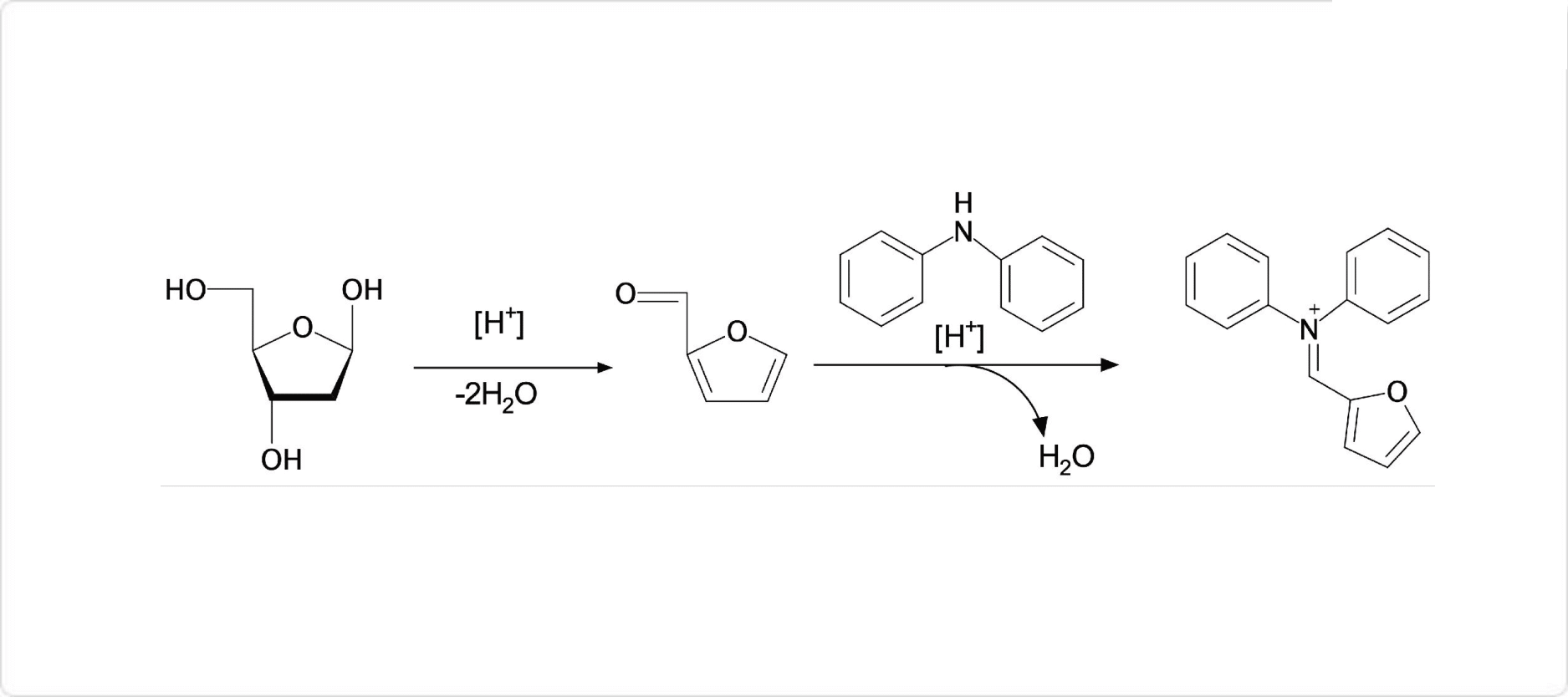
ディッシュ反応
ジフェニルアミンは、化学反応によって様々な色に呈色する物質です。濃硫酸溶液にジフェニルアミンと硝酸イオンまたは亜硝酸イオンを加える反応では濃青色に呈色します。この反応はルンゲ試験と呼ばれ、ルンゲ試験はこれらのイオンを検出するために用いられます。
また、2-デオキシペントースとジフェニルアミンの反応はディッシュ反応と呼ばれ、青色に呈色します。ディッシュ反応はDNAの定量分析に用いられています。
化学反応
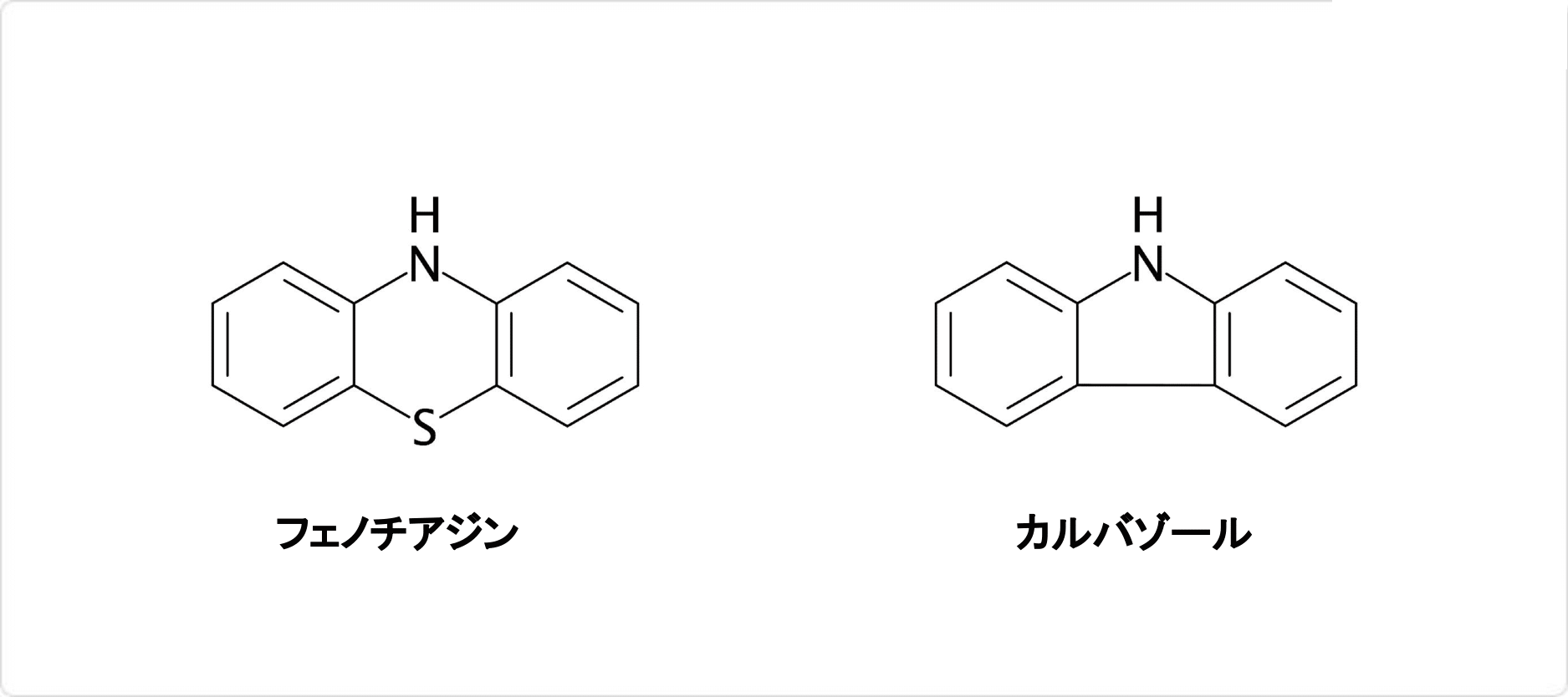
ジフェニルアミンの環化反応による生成物
ジフェニルアミンは弱い塩基であり塩基解離定数Kbは10−14です。強酸と反応して塩を生じ、例えば、硫酸との反応では白色もしくは淡黄色固体の [(C6H5)2NH2]+[HSO4]−を生じます。
また、ジフェニルアミンは容易に環化反応を起こす物質です。例えば、硫黄との反応ではフェノチアジンを生じ、ヨウ素との反応では脱水素化反応によりカルバゾールが生成します。
有害性
ジフェニルアミンは、重篤な眼の損傷、発がんの恐れ、生殖能又は胎児への悪影響の恐れ、中枢神経系・血液系の障害、呼吸器への刺激の恐れ、長期にわたる又は反復ばく露による血液系、腎臓の障害の恐れなどの有害性が指摘されている物質です。
労働安全衛生法では、名称等を表示し、又は通知すべき危険物及び有害物として指定されています。取り扱いの際は、適切な保護具を使用し、法令を遵守した正しい取り扱いを行うことが必要です。
また、可燃性の物質であるため消防法では、指定可燃物、可燃性固体類に指定されています。
农业用途
Insecticide, Fungicide, Herbicide, Plant growth
regulator: Topically in anti-screwworm mixtures, foliar application in a modified growth chamber to decrease ozone injury to
leaves of apple, bean, muskmelon, petunia, and tobacco plants.
To control weather fleck in tobacco and inhibit algae formation. To prolong the fresh appearance of snapdragons. Protect
rice from the toxic effects of thiolcarbamate herbicides [83] . Not
currently approved for use in EU countries (resubmitted) .
Registered for use in the U.S. and other countries.
製品名
NOSCALD DPA 31; NOSCALD DPA
283; SCALDIP; Z-876
安全性プロファイル
Poison by ingestion.
Experimental teratogenic effects. Action
similar to anhne but less severe.
Combustible when exposed to heat or
flame. Can react violently with
hexachloromelamine or trichloromelamine.
Can react with oxilzing materials. To fight
fire, use CO2, dry chemical. When heated to
decomposition it emits highly toxic fumes of
NOx,. See also ANILINE, AMINES, and
AROMATIC AMINES.
環境運命予測
Diphenylamine is present in waste water from industrial
processes. Diphenylamine has been detected in milk of
animals (cow, sheep, goat, water buffalo) raised in Italy and
France.
Pseudokirchneriella subcapitata (Algae) growth was inhibited
with a dose of 0.30 mg l
-1. Aquatic invertebrates Daphnia
magna showed an acute 48 h EC50 dose of 1.2 mg l
-1.
代謝経路
The major metabolite of diphenylamine (DPA)
identified in stored apples is a glucose conjugate of
4-hydroxydiphenylamine, and additional metabolites,
characterized as glycosyl conjugates of 2-hydroxy-
DPA, 3-hydroxy-DPA, 4-hydroxy-DPA, or dihydroxy-
DPA, are also detected along with their intact (i.e.
non-conjugated) forms in apple pulp.
貯蔵
Diphenylamine should be protected from physical damage. Storage of diphenylamine
outside or a detached area is preferred. Inside storage should be in a standard flammable
liquids storage room or cabinet. Diphenylamine should be kept separately from oxidizing
materials and incompatible chemical substances. Storage and work areas should be no
smoking areas. Diphenylamine should be kept protected from light.
純化方法
Crystallise diphenylamine from pet ether, MeOH, or EtOH/water. Dry it under vacuum. [Beilstein 12 H 174, 12 IV 271.]
予防処置
Students and occupational workers should be careful during use and handling of diphenylamine.
Workers should wear impervious protective clothing, including boots, gloves,
a laboratory coat, apron or coveralls, as appropriate, to prevent skin contact. Finely dispersed
particles of diphenylamine form explosive mixtures in air. Diphenylamine is very
harmful on exposures by swallowing, inhalation, and/or skin absorption. Diphenylamine
causes irritation to the skin, eyes, and respiratory tract, and causes blood vascular changes
leading to methemoglobinemia.
ジフェニルアミン 上流と下流の製品情報
原材料
準備製品
1,3-ジヒドロ-7-クロロ-5-フェニル-2H-1,4-ベンゾジアゼピン-2-チオン
メタニル エロー
タンプラミン
β-アラニン
N-[4-[[4-(ジメチルアミノ)フェニル][4-(エチルアミノ)ナフタレン-1-イル]メチレン]-2,5-シクロヘキサジエン-1-イリデン]-N-メチルメタンアミニウム·クロリド
5-[[4-(アミノスルホニル)-2-ニトロフェニル]アミノ]-2-[[4-[[4-(アミノスルホニル)-2-ニトロフェニル]アミノ]フェニル]アミノ]ベンゼンスルホン酸
Direct Blending Navy Blue D-R
N,N'-ジ-1-ナフチル-N,N'-ジフェニルベンジジン (昇華精製品)
ダイレクト ブラック22
Direct Blend Blue
α-アミルシンナムアルデヒド
フェノチアジン
9-メチルアクリジン
ノルダゼパム
4,4'-ビス(α,α-ジメチルベンジル)ジフェニルアミン
N,N'-ジフェニルベンジジン
4-ニトロソジフェニルアミン
9,10-ジヒドロ-9,9-ジメチルアクリジン
3-アミノプロピオニトリル
2,2'-[(2,4-ジヒドロキシ-1,3-フェニレン)ビス(アゾ-4,1-フェニレンイミノ)]ビス[5-ニトロベンゼンスルホン酸ナトリウム]
エリオ グリーン B
ジフェニルアミン-4-スルホン酸ナトリウム
4-ジアゾジフェニルアミンスルファート
4-[[4-[(4-ヒドロキシ-2-メチルフェニル)アゾ]フェニル]アミノ]-3-ニトロベンゼンスルホン酸ナトリウム
4-アミノ-3-[[4-[[4-[(2-アミノ-4-ヒドロキシフェニル)アゾ]フェニル]アミノ]-3-(ソジオオキシスルホニル)フェニル]アゾ]-5-ヒドロキシ-6-(フェニルアゾ)-2,7-ナフタレンジスルホン酸ジナトリウム
C.I.サルファーブルー7
lithium base grease
2-アミノ-5-ニトロベンゾフェノン
N-ニトロソジフェニルアミン
Sulphur Dark Blue 3R
N,N'-ジフェニル-N,N'-ジ(m-トリル)ベンジジン
1,1-ジフェニル-2-ピクリルヒドラジル フリーラジカル
Molybdenum disulfide lithium-based grease
photosensitive polyimide photoresist (PSPI)
N,N,N',N'-テトラフェニルベンジジン
4,4`-di(a-methylbenzyl)diphenylamine