ペルオキシジナトリウム 化学特性,用途語,生産方法
種類
過酸化ナトリウムは、主に研究開発用試薬製品や、産業用無機薬品として販売されています。研究開発用試薬製品としては、10g、25g、100g、500gなどの容量の種類があります。
吸湿性があり、酸化性が強いため、取り扱いや保管には注意が必要です。通常室温で保管可能な試薬製品として取り扱われています。なお、産業用としてはドラムなどの荷姿で販売されています。
性質
過酸化ナトリウムの無水物は、式量77.98、融点460℃、沸点675℃であり、常温常圧では淡黄色の粉末状結晶です。なお、八水和物は無色の結晶状固体です。
水に溶けやすく、その時酸素を発生して発熱します。また、エタノールやその他の可燃性物質に触れると発火します。密度は2.80g/mLです。
溶解性
水に易溶 (分解), エタノールに不溶。水に溶けやすく、その時酸素を発生して発熱する。エタノール又はその他の可燃性物質に触れると発火する。
解説
Na2O2(77.98).金属ナトリウムを二酸化炭素を含まない乾燥空気中で300 ℃ に熱して得られる.淡黄色の粉末.正方晶系.融点460 ℃.密度2.81 g cm-3.500 ℃ まで安定である.きわめて吸湿性で,水とはげしく反応して酸素を発生し,水酸化ナトリウムとなる.冷水または酸性水溶液では過酸化水素を生じる.強酸化剤でCO2と反応してNa2CO3と O2 を,COとではNa2CO3を生じる.強アルカリ性水溶液中で,CrⅢをCrO42-に酸化する.ケイ酸塩の融解酸化にも用いられる(過酸化物融解).融解したNa2O2はPtを侵すので,Ni,Au,またはAgのるつぼを用いる.硫黄,有機物と混合すると発火または爆発する.また,湿った空気中で粉末アルミニウム,炭と混合しても爆発する.酸化剤,漂白剤,殺菌,薬用せっけん,有機過酸化物の製造,分析試薬などに用いられる.密栓保存する.皮膚や粘膜をおかす.[CAS 1313-60-6]
森北出版「化学辞典(第2版)
用途
・酸化剤として動植物繊維、羽毛、骨、象牙、ワックスなどの漂白・過酸化物、過ホウ酸塩などの製造・分析試薬・二酸化炭素吸収剤として潜水艦などの空気浄化用・融解して小立方体に固めたものはオキソンという商品名で酸素発生剤
合成
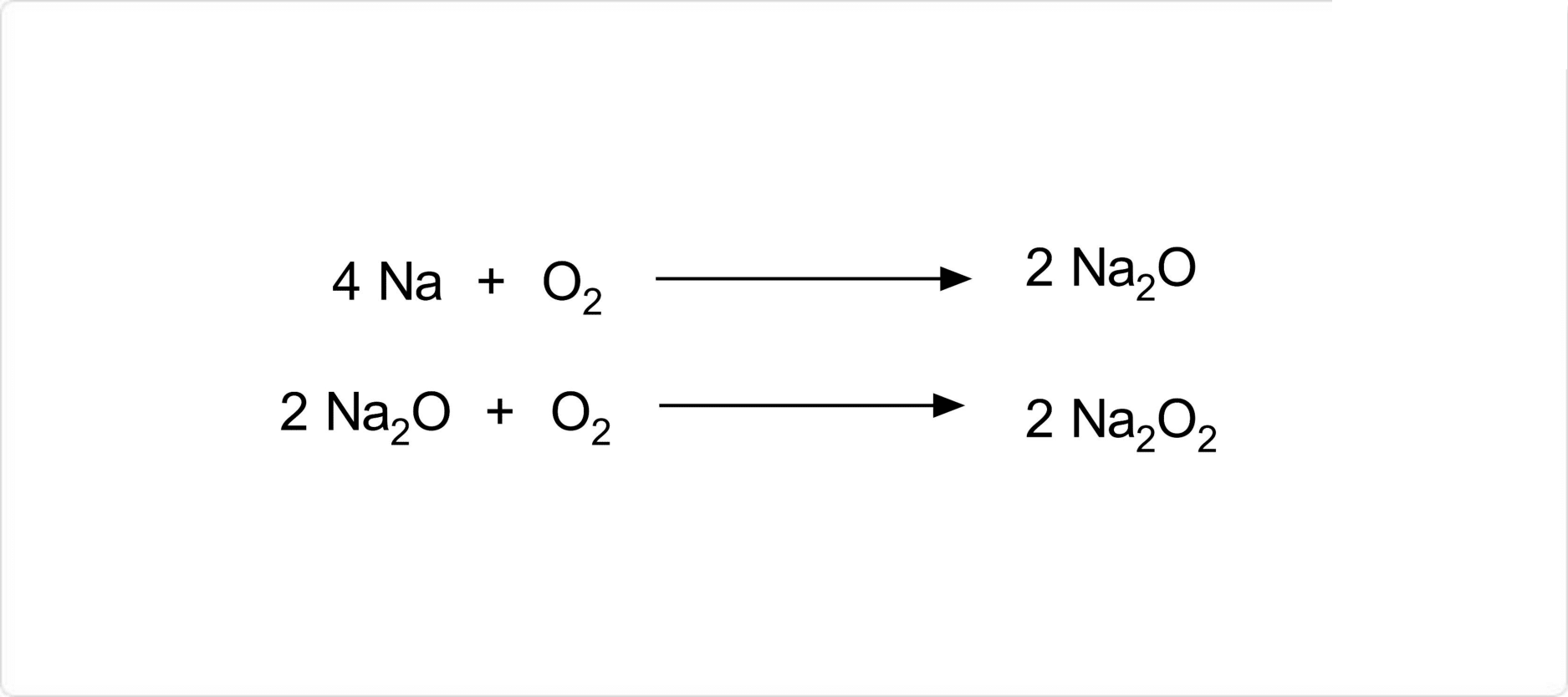
図. 過酸化ナトリウムの合成
金属ナトリウムを乾燥空気中で130℃から200℃の高温で加熱することにより、酸化ナトリウムを経由して過酸化ナトリウムの無水物を得ることができます。また、過酸化ナトリウムの八水和物は、過酸化水素と水酸化ナトリウム水溶液を混合することによって合成が可能です。
使用上の注意
強い酸化性があるので、有機物及び還元性物質との接触又は混合を避けて下さい。吸湿しやすいので、使用後は直ちに栓又は封をして下さい。
説明
Sodium peroxide has a molecular formula of Na2O2 and is an inorganic peroxide salt. It is a yellowish-white powder that turns yellow when heated. Sodium peroxide absorbs water and carbon dioxide from the air and is soluble in cold water. It is a strong oxidizing agent, is corrosive and can cause burns to the eyes and skin, and is also toxic by ingestion and inhalation. Sodium peroxide is water reactive and a dangerous fire and explosion risk in contact with water, alcohol, or acids. Sodium peroxide forms self-igniting mixtures with powdered metals and organic materials. It is incompatible with ethyl or methyl alcohol, glacial acetic acid, carbon disulfide, glycerin, ethylene glycol, and ethyl acetate. The four-digit UN identification number is 1504. The NFPA 704 designation is health 3, flammability 0, and reactivity 1. The 704 diamond has the prefix “oxy” in the white space at the bottom. It is used as bleach and as an oxygen-generating material for diving bells and submarines.
化学的特性
Sodium peroxide, Na202, is a fire-hazardous white powder that yellows when heated and causes ignition when in contact with water. Sodium peroxide is decomposed by heating, although this is not easily accomplished. It is stable in dry air; however, in moist air,or when acted on by water, it decomposes readily. It can be a powerful oxidizer and a powerful reducing agent, depending on conditions. Sodium peroxide is also used as a bleach, in medicine soap, and in the decomposition of minerals.
使用
Sodium peroxide historically was used to bleach wood pulp
for the production of paper and textiles. It is mainly used for
specialized laboratory operations, for example, the extraction
of minerals from various ores. Sodium peroxide is used
as an oxidizing agent and is used as an oxygen source by
reacting with carbon dioxide to produce oxygen and sodium
carbonate; it is thus particularly useful in scuba gear, submarines,
and so on.
定義
Exists as impurity
(about 10%) in sodium peroxide, obtained by heat-
ing sodium peroxide in oxygen, reacts with water
to yield hydrogen peroxide, oxygen, and sodium
hydroxide.
一般的な説明
A yellow-white to yellow granular solid. Mixtures with combustible material are readily ignited by friction, heat, or contact with moisture. May vigorously decompose under prolonged exposure to heat, causing the rupture of the containers.
空気と水の反応
Reacts vigorously with water, large amounts react explosively [Haz. Chem. Data 1969. p. 201].
反応プロフィール
Sodium peroxide reacts violently with reducing agents, combustible materials and light metals. Reacts exothermically and rapidly or even explosively with water to form a strong base (NaOH) and oxygen (O2) [Handling Chemicals Safely 1980 p. 854]. A mixture with ammonium persulfate can explode if subjected to friction (crushing in a mortar), if heated, or if a stream of gaseous carbon dioxide is passed over Sodium peroxide [Mellor 10:464 1946-47]. Reacts very vigorously with gaseous hydrogen sulfide; even in the absence of air, the reaction may be accompanied by flame [Mellor 10:132 1946-47]. An explosion results when gaseous carbon dioxide is passed over a mixture of Sodium peroxide with powdered magnesium [Mellor 2:490 1946-47] . Mixtures with acetic acid or acetic anhydride can explode if not kept cold [Von Schwartz 1918 p. 321]. Spontaneously flammable in contact with aniline, benzene, diethyl ether, or organic materials such as paper and wood. Mixtures with charcoal, glycerine, certain oils, and phosphorus burn or explode [Mellor 2:490 1946-47]. A mixture with calcium carbide (powdered) burst into flame when exposed to damp air and exploded when heated [Mellor 2:490 1946-47]. Decomposes, often violently in the presence of catalytic quantities of manganese dioxide [Mellor 2 Supp. 2:635 1961]. Mixing with sulfur monochloride leads to a violent reaction [Mellor 2 Supp. 2:634 1961]. Can react with and cause the ignition of fuels.
危険性
Dangerous fire and explosion risk in contact
with water, alcohols, acids, powdered metals, and
organic materials. Strong oxidizing agent. Keep dry.
Irritant.
健康ハザード
TOXIC; inhalation or contact with vapor, substance, or decomposition products may cause severe injury or death. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
火災危険
May ignite combustibles (wood, paper, oil, clothing, etc.). React vigorously and/or explosively with water. Produce toxic and/or corrosive substances on contact with water. Flammable/toxic gases may accumulate in tanks and hopper cars. Some may produce flammable hydrogen gas upon contact with metals. Containers may explode when heated. Runoff may create fire or explosion hazard.
使用用途
過酸化ナトリウムは抗菌性・漂白作用・脱臭作用などを持つ物質です。そのため、衣服や配管など様々な製品に対して漂白剤や洗剤として用いられています。動植物繊維、羽毛、骨、象牙、ワックスなどの漂白にも使用可能です。
産業用途としては、分析用試薬、過酸化物、過ホウ酸塩などの有機過酸化物製造にも用いられています。二酸化炭素吸収剤として潜水艦などの空気浄化にも用いられる物質です。
また、過酸化ナトリウムは酸素系漂白剤として知られており、衣料用洗剤としてだけではなく洗濯槽の掃除にも用いられることが多いです。安全性が高く、漂白剤特有の臭いがしないなどの特徴を持つため、一般家庭でも広く用いられています。
過酸化ナトリウムを主成分とした漂白剤は、粉末状で販売されていることが多いです。衣料用漂白剤として用いる場合は、洗濯機に少量の過酸化ナトリウムを入れて洗濯することで簡単に漂白効果・脱臭効果を得ることができます。
化学反応
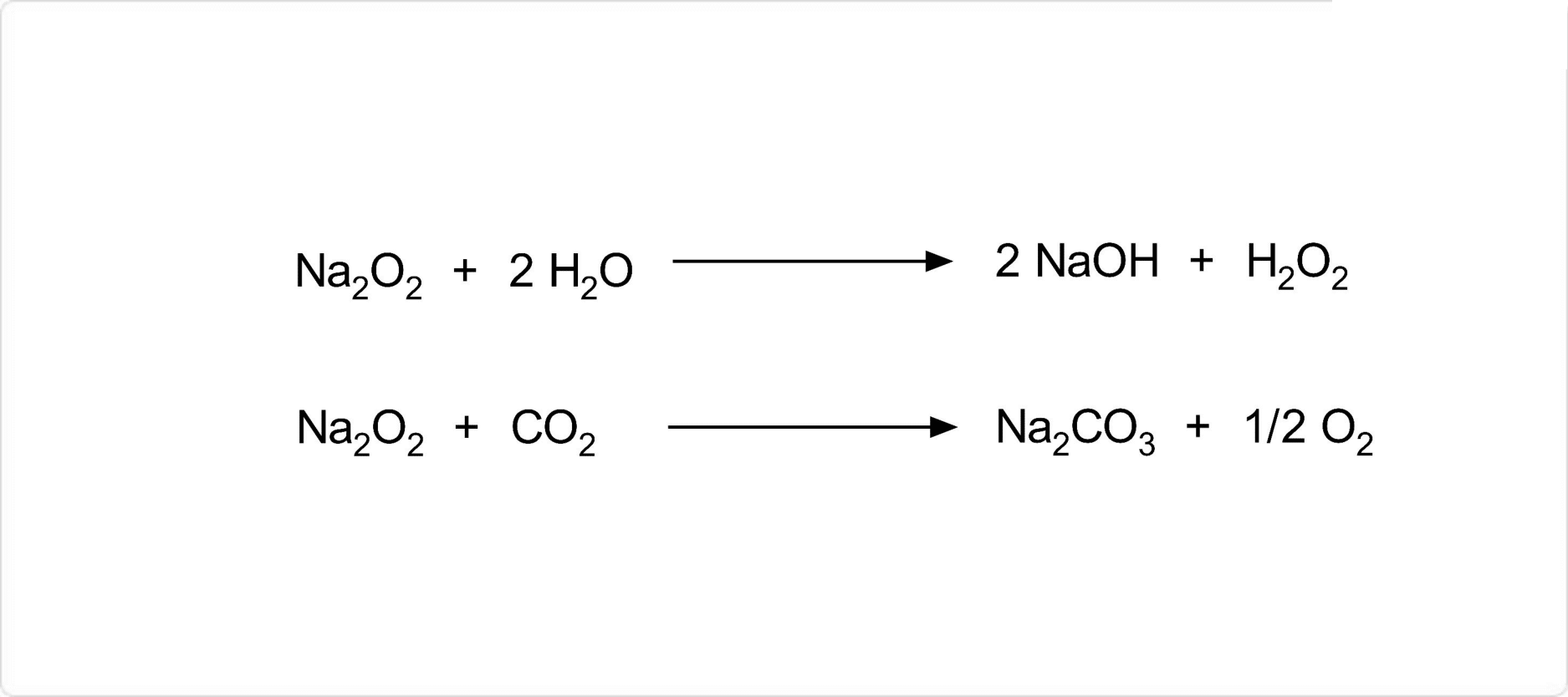
図. 過酸化ナトリウムの化学反応
過酸化ナトリウムは、水と激しく反応して水酸化ナトリウムと過酸化水素に分解します。また、沸点である657℃を超えて加熱すると酸素を放出して、酸化ナトリウムへ変化します。過酸化ナトリウムは強い酸化剤です。二酸化炭素を吸収して炭酸ナトリウムと酸素を生成し、一酸化炭素と反応して炭酸ナトリウムを生じます。
保管においては、湿気により発熱、発火することがあります。水と激しく反応し、有毒ガスを発生するため、水、湿気のある空気、火気を避けて保管することが必要です。また、 可燃物、有機物、金属粉との混触により発火又は爆発する恐れがあるため、こうしたものとの混触も避けるべきとされます。
法規制情報
過酸化ナトリウムは毒物及び劇物取締法で「劇物」に指定されている物質です。その他には、労働安全衛生法において「危険物・酸化性のもの」に指定されている他、消防法で「第1類酸化性固体、無機過酸化物」に指定されています。法令を遵守して正しく取り扱うことが重要です。
参考文献
安全性プロファイル
A severe irritant to shin, eyes, and mucous membranes. Dangerous fire hazard by chemical reaction; a powerfuloxidizing agent. Reacts explosively or violently under the appropriate conditions with water, acids, powdered metals, acetic acid, acetic anhydride, Al, (Al + CO2), aluminum + aluminum chloride, almond oil, (NH4)2S208, aniline, Sb, As, benzene, boron nitride, calcium aceqlide, charcoal, Cu, cotton wool, (KNO3 + dextrose), diethyl ether, fibrous materials + water, glucose + potassium nitrate, hexamethylene-tetramine, hydrogen sulfide, hydroxy compounds (e.g., ethanol, ethylene glycol, glycerol, sugar), magnesium, (Mg + CO2), MnO2, metals, metals + carbon dioxide + water, nonmetals (e.g., carbon, phosphorus, antimony, arsenic, boron, sulfur, selenium), nonmetal halides (e.g., diselenium dichloride, disulfur dichloride, phosphorus trichloride), organic matter, paraffin, K, silver chloride + charcoal, soap, Na, sodium dioxide, SCl, Sn, Zn, wood, peroxyformic acid, reducing materials. Will react with water or steam to produce heat and toxic fumes. To fight fire, use carbon dioxide or dry chemical. Combustible materials ignited by contact with sodium peroxide should be smothered with soda ash, salt or dolomite mixtures. Chemical fire extinguishers should not be used. If the fire cannot be smothered, it should be flooded with large quantities of water from a hose. When heated to decomposition it emits toxic fumes of Na2O. See also SODIUM HYDROXIDE and PEROXIDES, INORGANIC.
ペルオキシジナトリウム 上流と下流の製品情報
原材料
準備製品