ジメチルジスルフィド 化学特性,用途語,生産方法
外観
無色~黄褐色, 澄明の液体
種類
ジメチルジスルフィドは、主に研究開発用試薬製品として販売されています。容量の種類には、5mL、25mL、250mL、1Lなどがあります。
通常、室温で取り扱い可能な試薬製品です。主な用途は、機器分析等の標準調製液原料や有機合成原料などであり、研究開発用以外の用途で使用することはできません。
性質
ジメチルジスルフィドは、分子量94.19、融点-85℃、沸点110℃であり、常温における外観は黄色透明の液体です。ニンニクに似た強い硫黄臭があります。密度は1.06g/mLで、エタノール、エーテル、などの有機溶媒に容易に溶解します。水にはわずかに溶ける物質です。
反応性
ジメチルジスルフィドは、引火点が15℃と低く、引火性のある物質です。高温の表面、火花または裸火によって発火します。強酸化剤、強塩基、強還元剤と激しく反応する性質もあります。燃焼により、一酸化炭素、ニ酸化炭素、硫黄酸化物が発生する物質です。
ジメチルジスルフィドの塩素化反応によって、メタンスルフェニルクロリド (CH3SCl) や、メタンスルフィニルクロリド (CH3S(O)Cl) 、メタンスルホニルクロリド (CH3SO2Cl) が生成します。また、過酸化水素や過酢酸を用いた酸化反応による生成物は、硫黄原子が酸化された物質 (S-メチルメタンスルフィノチオエート: CH3S(O)SCH3) です。
溶解性
水に不溶。, エタノール, エーテル, アセトンに易溶。エタノール及びアセトンに極めて溶けやすく、水にほとんど溶けない。
解説
ジメチルジスルフィド,ニンニク臭の不快臭をもつ可燃性液体.融点-85 ℃,沸点108~109 ℃(100 kPa).d2041.0647.n20D1.5260.酸化によりメタンスルホン酸を与える.ごみ,し尿,下水などから産出し,天然精油などにも微量含まれている.悪臭防止法で指定されている悪臭物質.
森北出版「化学辞典(第2版)
用途
水添脱硫触媒用初期硫化剤、農薬中間体、硫黄の溶剤、チオメチル化剤、オニオン及びキャベツ系食品香料
用途
機器分析等の標準、調製液原料。
合成
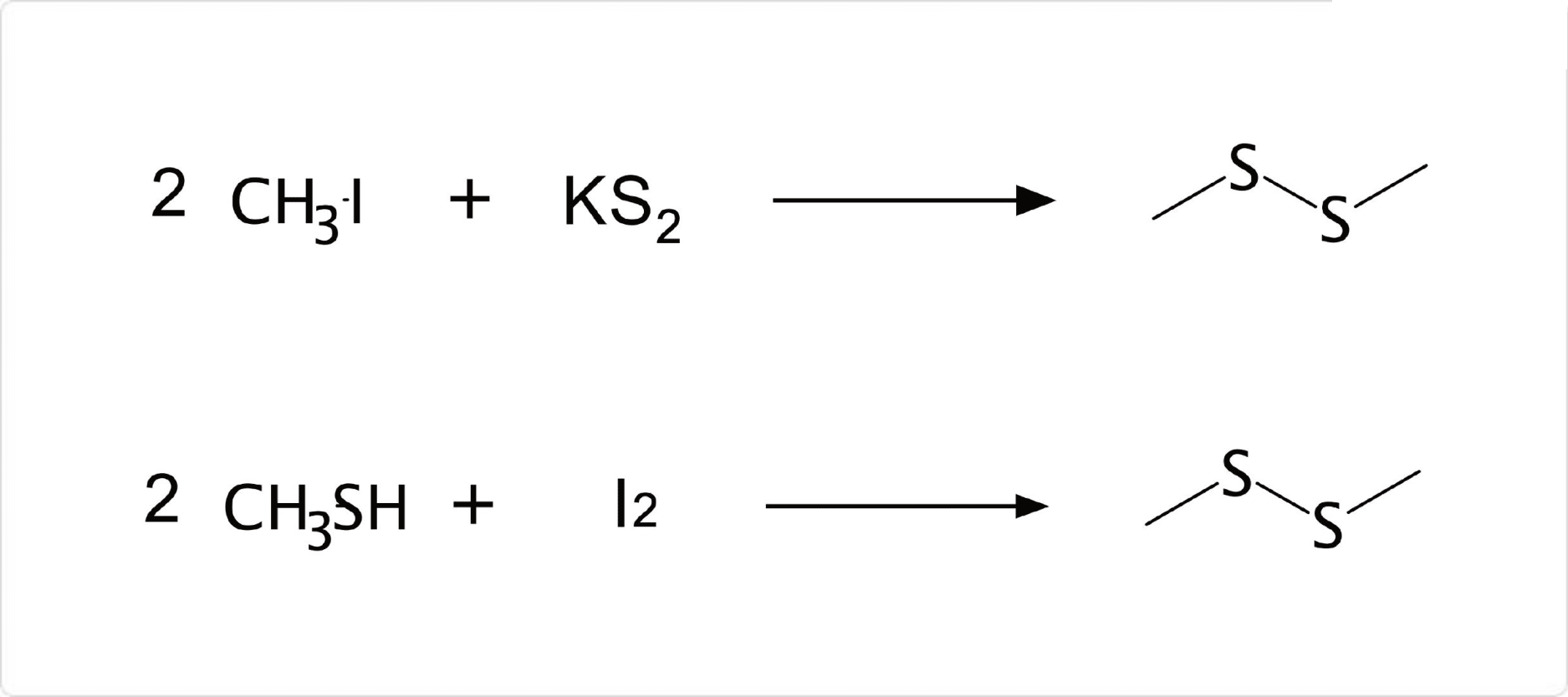
図2. ジメチルジスルフィドの合成
ジメチルジスルフィドは、と二硫化カリウムの反応や、ヨウ素を用いたメタンチオールの酸化反応によって合成が可能です。自然界ではコチなどの一部の魚や、アブラナ科の植物、ニンニクなどに含まれています。
特に腐敗すると誘導体の分解により発生するため、ごみ、し尿、下水などから産出されることも多くあります。天然精油などにも微量含まれる物質です。
製造
ジメチルジスルフィド,二硫化ジメチルともいう.ヨウ化メチルに二硫化カリウムを反応させると得られる.
説明
Dimethyl disulfide has a diffuse intense onion odor. It is nonlachrymatory. Ironically, although many find its odor objectionable at high concentrations as noted above, when diluted, its aroma has also been described as pleasant. Dimethyl disulfide is often used in combination with other flavor compounds in food products, including baked goods, cheese, frozen dairy products, meat products, soups, savory flavors, fruit flavors, soft candy, gelatin, puddings, and both alcoholic and nonalcoholic beverages.
化学的特性
colourless to yellow liquid with a very unpleasant smell, insoluble in water, Soluble in ethanol, ethyl ether and acetic acid. It is a strong odor component irritant which is also included in garlic and has been designated as a specific malodorous substances based on the Offensive Odor Control Law. Its detection threshold shows 0.002 ppm, which senses a low concentration equivalent to Dimethyl sulfide.
天然物の起源
Reported found in sour cherry, guava, melon, peach, pineapple, strawberry, cabbage, kohlrabi, onion, garlic,
shallot, leek, chive, peas, potato, rutabaga, tomato, parsley, breads, many cheeses, yogurt, milk, egg, fish, meats, hop oil, beer, Scotch
whiskey, cognac, grape wines, cocoa, coffee, peanut, peanut butter, pecan, potato chips, oats, soybean, beans, mushrooms, trassi,
macadamia nut, mango, cauliflower, broccoli, brussels sprouts, rice, radish, sukiyaki, sake, watercress, malt, wort, krill, southern
pea, loquat, sapodilla, shrimp, oyster, crab, crayfish, clam, scallops and squid.
使用
Dimethyl disulfide is used as an intermediate as well as a food additive in onion, garlic, cheese, meats, soups, savory flavors and fruit flavors. It is used in oil refineries as a sulfiding agent. It is used to alfa substitute 2-methylfuran-acrolein to produce food stuff. It is involved in the preparation of 4-(methylthio)phenol. Further, it is used to replace methyl mercaptan as a jet fuel additive. It finds application in artificial flavoring agent and a corrosion inhibitor. In addition to this, it serves as an effective soil fumigant in agriculture.
製造方法
Dimethyl disulfide can be prepared by the reaction between imethyl sulfate and sodium sulfide. under stirring, sulfur powder was added into sodium sulfide solution. The above reaction system was heated Up to 80-90℃, after reaction for 1 h, cooled to about 30 ℃. Dimethyl sulfate was dropped into the reaction system and the reaction was continued for 2h. Then, distillation, stratification, Separating waste alkali liquor, then through distillation and final products are prepared.
In industry,dimethyl sulfate method is adopted to synthesize dimethyl disulfide.
Na2S+S→Na2S2Na2S2+(CH3)2SO4→CH3SSCH3+Na2SO4
From magnesium methyl iodide and S2Cl2, or from S2S2 and sodium methyl sulfate; also from methyl bromide and sodium thiosulfate, after which the resulting sodium methylthiosulfate is heated to yield dimethyl disulfide.
定義
ChEBI: Dimethyl disulfide is an organic disulfide that is methane in which one of the hydrogens has been replaced by a methyldisulfanyl group. It has a role as a xenobiotic metabolite.
一般的な説明
A colorless oily liquid with a garlic-like odor. Denser than water and slightly soluble in water. Flash point 40°F. Vapors heavier than air. May irritate skin and eyes. Used to make other chemicals.
空気と水の反応
Highly flammable. Slightly soluble in water.
反応プロフィール
DMDS is a reducing agent. A dangerous fire hazard when exposed to oxidizing materials. Emits toxic fumes of oxides of sulfur when heated to decomposition or on contact with acids [Sax, 9th ed., 1996, p. 1320].
健康ハザード
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
火災危険
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
安全性と法規制情報
ジメチルジスルフィドは前述の通り引火点15℃の引火性のある物質です。そのため、消防法において第4類引火性液体、第一石油類 (非水溶性液体) に指定されています。
人体に対する有害性も報告されており、 急性毒性の可能性、皮膚刺激、眼刺激、発がんの可能性、中枢神経系・呼吸器系の障害のおそれ、長期又は反復ばく露による鼻粘膜・血液の障害のおそれなどが指摘されています。
労働安全衛生法では、 名称等を表示すべき危険有害物、リスクアセスメントを実施すべき危険有害物、危険物・引火性の物に指定されています。法令を遵守して正しく取り扱うことが必要な物質です。
参考文献
https://www.jstage.jst.go.jp/article/bunsekikagaku/54/11/54_11_1075/_pdf
使用用途
ジメチルジスルフィドの主な使用用途は、燃料油などの精製における水添脱硫触媒用初期硫化剤、農薬中間体、合成樹脂の製造過程における溶剤、チオメチル化剤などです。開発研究レベルでは、機器分析等の標準、調製液原料などにも用いられています。
また、ジメチルジスルフィドは、刺激性が強く、ニンニクに似た特有の硫黄臭を持ち、特定悪臭物質に指定されている物質です。一方で、タマネギやキャベツなどの食品用香料として使用されています。加熱調理においてはジメチルジスルフィドおよびジメチルスルフィドの増加により、煮沸臭あるいはキャベツ臭が増して不快に感じます。
安全性プロファイル
Poison by inhalation. A
very dangerous fire hazard when exposed to
heat, flame, or oxidzers. Can react
vigorously with oxiduing materials. See also
SULFIDES.
純化方法
Pass it through neutral alumina before use. [Trost Chem Rev 78 363 1978, Beilstein 1 IV 1281.]
ジメチルジスルフィド 上流と下流の製品情報
原材料
準備製品