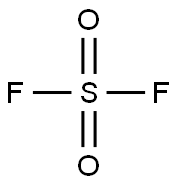
황화 불소 C화학적 특성, 용도, 생산
화학적 성질
Sulfuryl fluoride is a colorless, poisonous gas. Odorless.
용도
Sulfuryl fluoride is another promising chemical, which is widely used for control of termites, and stored-product insects. This chemical shows considerable promise and is undergoing registration procedures as replacement for methyl bromide in several countries including the United States, Great Britain, Italy, and France. The fumigant has vapour pressure of about ten times higher (13,442 mm of Hg at 25oC) than that of methyl bromide and hence it is more penetrative into treated commodities which is a significant benefit in situations where insect infestations are located deep within cracks or machinery in premises such as flour mills . The fumigant has several other advantages as a replacement for methyl bromide; it is nonreactive with structural materials and components of electronic equipment, is non flammable and has not caused problems of taint following treatment of a wide range of materials. However the sulfuryl fluoride was found to be less effective in controlling the insect eggs.
일반 설명
A colorless odorless gas. Shipped as a liquefied gas under its own vapor pressure. Noncombustible. Heavier than air. Very toxic by inhalation. Contact with the unconfined liquid can cause frostbite. Prolonged exposure to heat can cause containers to rupture violently and rocket.
공기와 물의 반응
Reacts with moist air to give corrosive acid mists that are heavier than air. Decomposes with water forming hydrofluoric acid and sulfuric acid. Reacts violently with bases [Handling Chemicals Safely p. 881 1980].
반응 프로필
SULFURYL FLUORIDE is incompatible with water, strong oxidizing agents, alcohols, amines, and bases. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
위험도
Toxic by inhalation. Central nervous system
impairment.
건강위험
TOXIC; may be fatal if inhaled or absorbed through skin. Vapors may be irritating. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
화재위험
Some may burn but none ignite readily. Vapors from liquefied gas are initially heavier than air and spread along ground. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
농업용
Fumigant: Sulfuryl fluoride is used to fumigate closed structures
and their contents such as domestic dwellings, garages,
barns, storage buildings, commercial warehouses,
ships in port, and railroad cars. It controls numerous insect
pests including termites, powder post beetles, old house
borers, bedbugs, carpet beetles, clothes moths and cockroaches,
as well as rats and mice. It is also used in organic
synthesis of drugs and dyes. Use pending in EU countries[
115]. Registered for use in the U.S.
상품명
TERMAFUME®; VIKANE®; VIKANE
FUMIGANT®
Safety Profile
Poison by ingestion. Mildly toxic by inhalation. Accidental human exposure caused nausea, vomiting, cramps, itching. May be narcotic in high concentration. Experimental reproductive effects. Can react with water, steam. When heated to decomposition it emits very toxic fumes of Fand SOx. See also FLUORIDES.
잠재적 노출
Sulfuryl fluoride is used an insecticidal fumigant. It is also used in organic synthesis of drugs and dyes.
운송 방법
UN2191 Sulfuryl fluoride, Hazard Class: 2.3; Labels: 2.3-Poisonous gas, Inhalation Hazard Zone D.
비 호환성
Reacts with water and steam; at temperatures >300℃, forms strong, highly corrosive acids. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from metal hydroxides, alcohols, alkaline materials, amines, strong acids, strong bases. Fluorides form explosive gases on contact with strong acids or acid fumes.
폐기물 처리
Return refillable compressed gas cylinders to supplier. Addition of soda ash-slaked lime solution to form the corresponding sodium and calcium salt solution. This solution can be safely discharged after dilution. The precipitated calcium fluoride may be buried or added to a landfill. Small amounts could also be released directly to the atmosphere without serious harm.
황화 불소 준비 용품 및 원자재
원자재
준비 용품