부데소나이드 C화학적 특성, 용도, 생산
개요
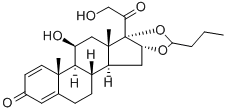
Budesonide is composed
of a 1:1 mixture of epimers of the 16,17-butylacetal, creating a chiral center. The 22R-epimer
binds to the GR with higher affinity than does the 22S-epimer (Table 33.5). The butyl acetal chain
provided the highest potency for the homologous acetal chains. Its rate of topical uptake into epithelial tissue is
more than 100 times faster than that for hydrocortisone and dexamethasone. Approximately 85% of the IV
administered dose of budesonide undergoes extensive first-pass hepatic metabolism by CYP3A4 to its primary
metabolites, 6β-hydroxybudesonide and 16α-hydroxyprednisolone, which have approximately 1/100 the potency
of budesonide. This is an important inactivation step in limiting budesonide's systemic effect on
adrenal suppression.
화학적 성질
White Solid
용도
Budesonide is a glucocorticoid steroid that activates the glucorcorticoid receptor with an EC50 value of 12.4 nM. Like other glucocorticoids, budesonide reduces inflammation and has utility in inflammatory diseases, like asthma and inflammatory bowel disease. Also like other glucocorticoids, budesonide may be abused by athletes.[Cayman Chemical]
정의
ChEBI: A glucocorticoid steroid having a highly oxygenated pregna-1,4-diene structure. It is used mainly in the treatment of asthma and non-infectious rhinitis and for treatment and prevention of nasal polyposis.
Indications
Budesonide is a synthetic corticosteroid having a
potent glucocorticoid and weak mineralocorticoid activity.
In standard in vitro and animal models, budesonide
has an approximately 200-fold higher affinity for
the glucocorticoid receptor and a 1000-fold higher topical
antiinflammatory potency than cortisol.
일반 설명
Budesonide (Pulmicort Turbohaler,Rhinocort) is extensively metabolized in the liver, with 85%to 95% of the orally absorbed drug metabolized by the firstpasseffect. The major metabolites are 6β-hydroxybudesonideand 16α-hydroxyprednisolone, both with less than1% of the activity of the parent compound. Metabolism involvesthe CYP3A4 enzyme, so coadministration of budesonidewith a known CYP3A4 inhibitor should be monitoredcarefully.
생물학적 활성
Synthetic anti-inflammatory glucocorticoid that displays chemopreventive activity. Prevents formation of lung adenomas and adenocarcinomas in mice following inhalation or oral administration. Reverses DNA hypomethylation and modulates expression of cancer related genes.
Mechanism of action
Budesonide is an acetal formed between the 16α,17α-dihydroxyl groups and butanal. It is a nonhalogenated glucocorticoid with a 16,17-acetal that decreases the mineralocorticoid activity. In receptor affinity studies, the R-epimer was twofold more active than the S-epimer. Because the C-21 hydroxy is free, budesonide is not a prodrug and is active as administered. Only 34% of the metered dose of inhaled budesonide reaches the lung.
Pharmacology
While budesonide is well absorbed from the GI tract, its oral
bioavailability is low (about 10%), primarily because
of extensive first-pass metabolism in the liver. Two major
metabolites (16α-hydroxyprednisolone and 6β-
hydroxybudesonide) are formed via the cytochrome
P450 3A enzyme. In vitro studies on the binding of the
two primary metabolites to the corticosteroid receptor
indicate that their affinity for the receptor is less than
1% of that of the parent compound. It is hoped that use
of this drug will avoid the long-term adverse reactions
seen with systemically active corticosteroids.
Clinical Use
Recently, budesonide (Entecort EC) has been approved
for the treatment of mildly to moderately active
Crohn’s disease involving the ileum and/or ascending
colon.
신진 대사
Budesonide was metabolized three- to sixfold more rapidly than triamcinolone acetonide.
The pharmacokinetics of budesonide after inhalation, oral, and IV administration displayed a mean plasma
half-life of 2.8 hours and a systemic bioavailability of approximately 10% after oral administration (Table 33.5)
(101). Pulmonary bioavailability is less than 40% after inhalation (70–75% after correction for the amounts of
budesonide deposited in the inhalation device and oral cavity). No oxidative metabolism was observed in the
lung. When given by inhalation, 32% of the dose is excreted in the urine as metabolites, 15% in the feces, and
41% of the dose remained in the mouthpiece of the inhaler. Following intranasal administration, very little of
intranasal budesonide is absorbed from the nasal mucosa. Much of the intranasal dose (~60%) was swallowed,
however, and remained in the GI tract to be excreted unchanged in the feces, whereas that fraction of the
intranasal dose that was absorbed was extensively metabolized.
부데소나이드 준비 용품 및 원자재
원자재
준비 용품