이산화티타늄
|
|
이산화티타늄 속성
- 녹는점
- 1840 °C
- 끓는 점
- 2900 °C
- 밀도
- 4.26 g/mL at 25 °C(lit.)
- 굴절률
- 2.61
- 인화점
- 2500-3000°C
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- 물에 거의 녹지 않습니다. 묽은 무기산에는 녹지 않으나 뜨거운 진한 황산에는 천천히 녹는다.
- 물리적 상태
- 가루
- 색상
- 흰색에서 약간 노란색
- Specific Gravity
- 4.26
- 수소이온지수(pH)
- 7-8 (100g/l, H2O, 20℃)(slurry)
- 냄새
- 100.00%. 냄새 없는
- 수용성
- 불용성
- Crystal Structure
- Orthorhombic, Pcab
- Merck
- 14,9472
- 노출 한도
- ACGIH: TWA 10 mg/m3
OSHA: TWA 15 mg/m3
NIOSH: IDLH 5000 mg/m3; TWA 2.4 mg/m3; TWA 0.3 mg/m3
- Dielectric constant
- 2.9(20℃)
- CAS 데이터베이스
- 13463-67-7(CAS DataBase Reference)
- IARC
- 2B (Vol. 47, 93) 2010
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | |||
|---|---|---|---|
| 위험 카페고리 넘버 | 10-20-22 | ||
| 안전지침서 | 26-36-25-2-36/37-45-36/37/39 | ||
| WGK 독일 | - | ||
| HS 번호 | 28230000 | ||
| 유해 물질 데이터 | 13463-67-7(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: > 10000 mg/kg | ||
| IDLA | 5,000 mg/m3 | ||
| 기존화학 물질 | KE-33900 |
이산화티타늄 C화학적 특성, 용도, 생산
물성
백색의 분말로서 냄새와 맛이 없다. 건조한 다음 정량할 때, 이산화티타늄(TiO2) 99.0% 이상을 함유한다. 무색 또는 백색 분말로 물에 불용이며 뜨거운 농황산 이외의 산에는 녹지 않는 음폐력이 매우 큰 색소이다. 화학식은 TiO2이다. 전이금속인 티타늄 원자 하나와 산소 원자 2개가 결합된 분자로서 분자량은 79.866g/mol이며, 무미, 무취의 흰색 가루이다. 티타늄을 공기 중에 노출시키면 쉽게 산소와 반응하여 이산화 티타늄 피막을 형성한다. 산화력이 매우 크며 음폐력이 커서 거의 모든 용매에 녹지 않으며 매우 안정한 물질이다. 생물학적으로 반응을 하지 않아 환경 및 인체에 무해하다. 판티탄석(brookite), 예추석(anatase), 금홍석(rutile)의 동질다상 형태로 존재한다. 산화력이 커서 향균 작용이 크고, 악취제거 및 살균작용이 있다.용도
이산화티타늄의 사용기준 이산화티타늄은 착색의 목적이외에 사용하여서는 아니되며, 아래의 식품에 사용하여서는 아니된다. 1. 면류2. 단무지
3. 특수용도식품(정제의 제피 또는 캡슐은 제외)
4. 건강기능식품(정제의 제피 또는 캡슐은 제외)
5. 유가공품(아이스크림류, 아이스크림분말류, 아이스크림믹스류 제외)
6. 두유류
7. 발효음료류
8. 과일·채소류음료(과·채음료 제외)
9. 인삼․홍삼음료
10. 두부류 또는 묵류
11. 젓갈류
12. 김치류
13. 절임식품(밀봉 및 가열살균 또는 멸균처리한 절임제품은 제외)
14. 조림식품
15. 천연식품[식육류, 어패류(고래고기포함), 채소류, 과실류, 해조류, 두류 등 및 그 단순가공품
(탈피, 절단 등)]
16. 벌꿀
17. 장류
18. 식초
19. 소스류
20. 토마토케첩
21. 잼류
22. 고춧가루 또는 실고추
23. 천연향신료
24. 카레
25. 식육가공품(소시지류, 식육추출가공품 제외)
26. 알가공품
27. 어육연제품(어육소시지 제외)
28. 식용유지류
29. 다류
30. 식빵
31. 마요네즈
32. 카스텔라
33. 레토르트식품
34. 즉석조리식품
35. 복합조미식품
36. 코코아버터
37. 땅콩 및 건과류가공품
38. 코코아분말
39. 조미김
40. 커피
공업용 용도로는 인쇄잉크, 화장품, 물감, 크레용, 제지, 건축용 메지, 비누, 아스팔트 타일, 페인트, 플라스틱, 제지산업 등에 사용된다.
순도시험
(1) 물가용물 : 이 품목 4g을 50mL의 물에 잘 흔들어 섞고 24시간 방치한 다음 200mL의 메스플라스크에 옮기고 염화암모늄시액 2mL를 넣어 섞는다. 이산화티타늄이 잘 침강하지 않을 때에는 다시 염화암모늄시액 2mL를 추가한다. 현탁액을 침강시키고 물을 넣어 정확히 200mL로 하고 잘 흔들어 섞으면서 여과하고 처음 10mL의 여액은 버리고 100mL의 맑은 여액을 모아서 미리 무게를 달아둔 백금접시에 옮기고 수욕상에서 증발건고한 다음 항량이 될 때까지 강열할 때, 그 잔류물은 5mg 이하이어야 한다(0.25% 이하).
(2) 염산가용물 : 이 품목 5g에 염산(1→20) 100mL를 가하여 흔들어 섞고 수욕상에서 30분간 때때로 흔들어주면서 가열한 다음 여과한다. 잔류물을 염산(1→20) 10mL씩으로 3회 씻고 세액을 여액에 합쳐서 증발건고한 다음 항량이 될 때까지 강열할 때, 그 잔류물은 25mg 이하이어야 한다(0.5% 이하).
(3) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 1.3ppm 이하이어야 한다.
(4) 납 : 이 품목 10.0g을 정밀히 달아 비이커에 넣고 0.5N 염산 50mL를 가해 주고 시계접시를 덮은 후 15분간 끓여준다. 식힌 다음 100~150mL 원심분리튜브에 옮기고 불용성물질이 가라앉을 때까지 10~15분 원심분리한 다음 상등액을 여과지(Whatman No. 4 또는 이와 동등한 것)로 여과한 후 여액을 100mL 플라스크에 옮긴다. 잔류물에 뜨거운 물 10~15mL로 가하고 섞어준 다음 원심분리하고 상등액을 여과하여 여액에 합한다. 이 조작을 2번 더 반복한 다음 여액에 합하고 물을 가하여 100mL로 한 액을 시험용액으로 하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 10.0ppm 이하이어야 한다.
(5) 카드뮴 : 순도시험 (4)의 시험용액을 사용하 여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(6) 안티몬 : 순도시험 (4)의 시험용액을 사용하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 2.0ppm 이하이어야 한다.
(7) 아연 : 순도시험 (4)의 시험용액을 사용하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 50ppm 이하이어야 한다.
(8) 수은 : 이 품목을 수은시험법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(9) 산화알루미늄 및 이산화규소 : 다음의 각각의 방법에 따라 산화알루미늄 및 이산화규소의 함량을 구할 때, 그 합계량은 2.0% 이하이어야 한다.
(i) 산화알루미늄 : 이 품목 1g 및 황산수소나트륨 10g을 취하여 석영제의 삼각플라스크에 넣고 완전하게 융해될 때까지 은근하게 가열한 다음 식히고 이에 황산(1→2) 25mL를 가해주고 침전이 녹을 때까지 주의깊게 가열하고 식힌 후 물을 가하여 120mL로 한다. 이 액에 수산화나트륨용액(1→4) 65mL를 흔들어 섞으면서 가해준 다음 미리 수산화나트륨용액(1→4) 135mL를 넣어준 500mL의 메스플라스크에 서서히 흔들어 섞으면서 가해주고 다시 물을 가하여 500mL로 한 다음 방치 또는 5분간 원심분리하고 여과한다. 여액 100mL를 500mL의 삼각플라스크에 넣고 메틸오렌지시액 1방울을 가해주고 염산(1→2)으로 산성으로 하고 다시 염산(1→2) 3mL를 더 가해준 다음 0.02M 이.디.티.에이.용액 25mL를 정확하게 가하여 액의 색이 적색에서 등황색으로 변할 때까지 암모니아시액을 적가한 다음 초산암모늄완충액(초산암모늄 77g에 빙초산 10mL를 가해주고 물을 가하여 1,000mL로 한 액) 10mL 및 인산이암모늄완충액(인산이암모늄 150g을 물 700mL에 녹인 액을 염산(1→20)으로 pH 5.5로 맞춘 다음 물을 가하여 1,000mL로 한 액) 10mL를 가해준다. 이어서 5분간 끓여준 다음 재빨리 흐르는 물에서 실온으로 냉각시킨 다음 자일레놀오렌지시액 3방울을 가해주고 혼합한다. 만일 액의 색이 자색, 황갈색 또는 홍색을 나타내는 경우에 있어서는 초산을 가하여 pH를 5.3~5.7로 한 다음에 홍색을 나타내지 않을 때에 이것을 시험용액으로 하고 홍색을 나타낼 때에는 이.디.티.에이.용액이 충분하지 않는 것을 의미하므로 앞의 여액 100mL를 다시 사용하여 동일조작을 행한 다음 시험용액으로 한다. 단, 0.02M 이.디.티.에이.용액은 25mL 대신에 50mL를 정확하게 취하여 사용한다. 이 시험용액을 0.01M 황산아연용액으로 액의 색이 황갈색에서 적색을 5~10초간 나타낼 때까지 적정한다.
(※ 주의 : 이 적정은 재빨리 수행되어야 하며 종말점 부근에서는 0.2mL씩 첫번째 색이 변할 때까지 가해주며 그 색이 5~10초안에 사라지더라도 종말점으로 한다. 첫번째 색의 변화관찰에 실패하면 부정확한 적정이 된다. 첫번째 적정소비 mL수는 8mL 이상이 되어야 하며 좀더 정확한 적정소비 mL수는 10~15mL이다.)
이 액에 불화나트륨 2g을 가하여 2~5분간 끓여준 다음 흐르는 물에서 급냉하여 유리된 이.디.티.에이.를 0.01M 황산아연용액으로 액의 색이 황갈색에서 적색을 나타낼 때까지 적정하고 다음 계산식에 따라 산화알루미늄의 함량을 구한다.
단, T는 아래의 방법에 따라 구하며 0.01M 황산아연용액 1mL에 대응하는 산화알루미늄 (Al2O3)의 양(mg)이다.
0.01M 황산아연용액 : 황산아연(ZnSO4‧7H2O) 2.9g을 물에 녹여 1,000mL로 한다. 알루미늄(고순도, 99.0%) 500mg을 정밀히 달아 염산 20mL를 가해주고 충분히 녹을 때까지 은근하게 가열해 주고 나서 물을 가하여 1,000mL로 한다. 이 액 10mL를 물 90mL 및 염산 3mL가 들어 있는 500mL 삼각플라스크에 넣고 메틸오렌지시액 한 방울 및 0.02M 이.디.티.에이.용액 25mL를 가해주고 나서 암모니아시액을 액의 적색이 등황색으로 될 때까지 적가한 다음 초산암모늄완충액 10mL 및 인산이암모늄완충액 10mL를 가하여 5분간 끓이고 급냉한 다음, 자일레놀오렌지시액 3방울을 가해주고 혼합한 후 이 황산아연용액을 액의 황색이 적색을 띨 때까지 가해주고 이어 불화나트륨 2g을 가하여 2~5분간 끓여준 다음 급냉하고, 유리된 이.디.티.에이.를 이 황산아연용액으로 액의 황색이 적색을 띨 때까지 적정하고 다음 계산식에 따라 T를 구한다.
W : 알루미늄 채취량(g)
V : 두번째 적정에서 소비된 황산아연용액의 적정량(mL)
(ii) 이산화규소 : 이 품목 1g 및 황화수소나트륨 10g을 취하여 백금제의 도가니에 넣고 완전하게 융해될 때까지 은근하게 가열한 다음 식히고, 이에 황산(1→2) 25mL를 가해주고 침전이 녹을 때까지 주의 깊게 가열하고 식힌 후 물 150mL를 서서히 흔들어 섞으면서 가해준 다음 정량분석용여지(5종C)를 사용하여 여과하고 사용한 도가니를 황산(1→2)으로 씻고 동일의 여지를 사용하여 여과한다. 이 여지를 별도의 백금제도가니에 넣고 120℃로 건조한 다음 주의하면서 450~550℃로 강열한다. 이어서 1,000℃에서 30분간 강열하고 데시케이타 중에서 방냉하고 전량을 정밀하게 달은 다음 그 양을 W(g)로 한다. 다음에 황산(1→2) 2방울 및 불화수소산 5mL를 가하여 서서히 가열하고 증발건고시킨 다음 1,000℃에서 30분간 강열하고 데시케이타 중에서 방냉하고 전량을 정밀히 달은 다음 그 양을 w(g)로 하고 다음 계산식에 따라 함량을 구한다.
확인시험
이 품목 약 0.5g에 황산 5mL를 넣어 흰 연기가 날 때까지 가열하고 식힌 다음 주의하여 물을 넣어 100mL로 하여 여과한다. 여액 5mL에 과산화수소시액 2~3방울을 넣을 때, 액은 황적색을 나타낸다.
정량법
이 품목을 건조한 다음 약 0.2g을 정밀히 달아 도가니에 넣고 이에 황산수소나트륨 2g을 가하여 뚜껑을 하고 완전히 융해될 때까지 은근하게 가열한 다음 다시 고온으로 내용물의 진한 구리색이 거의 징명한 색이 될 때까지 강열한다. 식힌 다음 내용물을 250mL의 비이커에 넣은 다음 사용한 도가니를 황산(1→30) 75mL로 씻고 세액을 앞의 비이커에 합한 다음 수욕상에서 거의 징명하게 녹을 때까지 가열한다. 이어서 주석산 2g을 가하고 브로모티몰블루시액 2~3방울을 가한 다음 암모니아시액으로 중화하고 필요하면 여과한다. 이 액에 황산(1→2) 1~2mL를 가하여 산성으로 하고 충분한 양의 황화수소를 통과시키고 이어서 암모니아시액 30mL를 가한다음 포화가 될 때까지 황화수소를 통과시키고 10분간 방치하고 여과한 다음 여지상의 침전을 주석산암모늄용액(1→100)․황화암모늄시액의 혼액(9 : 1) 2mL씩으로 10회 씻는다. 여과 및 세정시 여지는 액으로 항상 가득 채운다. 세액을 여액에 합해주고 이 액에 황산(1→2) 40mL를 가하여 황화수소가 제거될 때까지 끓여주고 식힌 다음 물을 가하여 400mL로 하고 쿠페론시액 40mL를 흔들어 섞어주면서 서서히 가하여 방치하고 황색의 침전이 침강된 후 다시 백색의 침전이 생길 때까지 쿠페론시액을 가해준다. 이 침전을 정량분석용여지(5종 C)를 사용하여 가볍게 흡인여과하고 염산(1→10)으로 20회 씻어준 후 약간 강하게 흡인하여 수분을 제거한다. 잔류물은 여지와 함께 70℃에서 건조하고 미리 무게를 달아둔 도가니에 넣고 연기가 발생하지 않을 때까지 극히 약하게 가열한 후 서서히 강열하여 900~950℃에서 항량이 될 때까지 강열하고 식힌 다음 무게를 평량하여 잔류물의 양을 구해서 W(g)로 하고 순도시험 (5)의 값을 이용하여 다음 계산식에 따라 함량을 구한다.
개요
Titanium dioxide, TiO2, is a white powder and has the greatest hiding power of all white pigments. It is noncombustible; however, it is a powder and, when suspended in air, may cause a dust explosion if an ignition source is present. It is not listed in the DOT Hazardous Materials Table, and the DOT does not consider it hazardous in transportation. The primary uses are as a white pigment in paints, paper, rubber, and plastics; in cosmetics; in welding rods; and in radioactive decontamination of the skin.화학적 성질
Ttitanium dioxide is an odorless white powder.물리적 성질
The naturally occurring dioxide exists in three crystal forms: anatase, rutile and brookite. While rutile, the most common form, has an octahedral structure. Anatase and brookite have very distorted octahedra of oxygen atoms surrounding each titanium atom. In such distorted octahedral structures, two oxygen atoms are relatively closer to titanium than the other four oxygen atoms. Anatase is more stable than the rutile form by about 8 to 12 kJ/mol (Cotton, F.A., Wilkinson, G., Murillo, C.A and M Bochmann. 1999. Advanced Inorganic Chemistry, 6th ed, p. 697, New York: John Wiley & Sons) Other physical properties are: density 4.23g/cm3; Mohs hardness 5.8 g/cm3 ( anatase and brookite) and 6.2 g/cm3 ( rutile); index of refraction 2.488 (anatase), 2.583 (brookite) and 2.609 (rutile); melts at 1,843°C; insoluble in water and dilute acids; soluble in concentrated acids.물리적 성질
Metastable over long periods of time despite being less thermodynamically stable than rutile. However, above 700°C, the irreversible and rapid monotropic conversion of anatase to rutile occurs. It exhibits a greater transparency in the near-UV than rutile. With an absorption edge at 385 nm, anatase absorbs less light at the blue end of the visible spectrum and has a blue tone.출처
Titanium dioxide occurs in nature in the crystalline forms rutile, anatase, and brookite. Rutile and anatase are manufactured in large quantities, which are primarily used as pigments, but also as catalysts and in ceramics.용도
Titanium dioxide is an extreme white and bright compound with high index of refraction. In paints it is a white pigment and an opacifying agent.It is in house paints, water paints, lacquers, enamels, paper filling and coating, rubber, plastics, printing ink, synthetic fabrics, floor coverings, and shoe whiteners. Also, it is used in colorants for ceramics and coatings for welding rods. A rutile form of the dioxide is used in synthetic gem stones.제조 방법
Titanium dioxide is mined from natural deposits. It also is produced from other titanium minerals or prepared in the laboratory. Pigment-grade dioxide is produced from the minerals, rutile and ilmenite. Rutile is converted to pigment grade rutile by chlorination to give titanium tetrachloride, TiCl4. Anhydrous tetrachloride is converted back to purified rutile form by vapor phase oxidation.Anatase form is obtained by hydrolytic precipitation of titanium(IV) sulfate on heating. The mineral ilmenite is treated with concentrated sulfuric acid. Heating the sulfate solution precipitates hydrous titanium oxide. The precipitate is calcined to expel all water.
Titanium dioxide also can be prepared by heating Ti metal in air or oxygen at elevated temperatures.
생산 방법
Titanium dioxide occurs naturally as the minerals rutile (tetragonal structure), anatase (tetragonal structure), and brookite (orthorhombic structure).Titanium dioxide may be prepared commercially by either the sulfate or chloride process. In the sulfate process a titanium containing ore, such as ilemenite, is digested in sulfuric acid. This step is followed by dissolving the sulfates in water, then precipitating the hydrous titanium dioxide using hydrolysis. Finally, the product is calcinated at high temperature. In the chloride process, the dry ore is chlorinated at high temperature to form titanium tetrachloride, which is subsequently oxidized to form titanium dioxide.
일반 설명
Two main physico-chemically distinct polymorphs of TiO2 are anatase and rutile. Anatase has a higher photocatalytic activity than rutile but is thermodynamically less stable.위험도
Lower respiratory tract irritant. Possible carcinogen.건강위험
Titanium dioxide is a mild pulmonary irritant and is generally regarded as a nuisance dust.Pharmaceutical Applications
Titanium dioxide is widely used in confectionery, cosmetics, and foods, in the plastics industry, and in topical and oral pharmaceutical formulations as a white pigment.Owing to its high refractive index, titanium dioxide has lightscattering properties that may be exploited in its use as a white pigment and opacifier. The range of light that is scattered can be altered by varying the particle size of the titanium dioxide powder. For example, titanium dioxide with an average particle size of 230nm scatters visible light, while titanium dioxide with an average particle size of 60nm scatters ultraviolet light and reflects visible light.
In pharmaceutical formulations, titanium dioxide is used as a white pigment in film-coating suspensions, sugar-coated tablets, and gelatin capsules. Titanium dioxide may also be admixed with other pigments.
Titanium dioxide is also used in dermatological preparations and cosmetics, such as sunscreens.
Safety Profile
A nuisance dust. A human skin irritant. Questionable carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. Violent or incandescent reaction with metals at high temperatures (e.g., aluminum, calcium, magnesium, potassium, sodium, zinc, lithium). See also TITANIUM COMPOUNDS.Safety
Titanium dioxide is widely used in foods and oral and topical pharmaceutical formulations. It is generally regarded as an essentially nonirritant and nontoxic excipient.잠재적 노출
Titanium dioxide is a white pigment used as a pigment in paint; in the rubber, plastics, ceramics, paint, and varnish industries, in dermatological preparations; and is used as a starting material for other titanium compounds; as a gem; in curing concrete; and in coatings for welding rods. It is also used in paper and cardboard manufacture.Carcinogenicity
Carcinogenesis. In a 1985 study, rats (CD) were exposed to graded airborne concentrations (0, 10, 50, and 250mg/m3) of TiO2 6 h/day, 5 days/week, for 2 years. The majority of the particles were in the respirable range (84% ≤13 mmMMD). All responses were confined to the lungs. At the lowest dose, the histopathological evaluation of the lungs revealed dust-laden macrophages in the alveolar ducts and adjacent alveoli with pneumocyte hyperplasia. At the two highest concentrations, there were increases in lung weight, accumulation of dust in the macrophages, foamy macrophage responses, type II pneumocyte hyperplasia, alveolar proteinosis, alveolar bronchiolization, cholesterol granulomas, focal pleurisy, and dust deposition in the tracheobronchiolar lymph nodes. At the 250mg/m3 exposure concentration, bronchiole alveolar adenomas (males: control 2/79, 250mg/m3 12/79; females: control 0/79, 250mg/m3 13/79) increased. Additionally, 13/79 females at the 250mg/m3 dose showed squamous cell carcinoma, compared with none in 79 controls. Theauthorsnoted that this responsemight have little biological relevance to humans because of the overload of respiratory clearance mechanisms and also pointed out that the type, location, and development of the tumors were different from those in human lung tumors. It is not clear that the nasal cavity epithelium was examined. However, the nasal cavity load would be expected to be higher in the rats because of anatomic structure, whereas the lung deposition should be higher in humans because we are, in part, mouth breathers.저장
Titanium dioxide is extremely stable at high temperatures. This is due to the strong bond between the tetravalent titanium ion and the bivalent oxygen ions. However, titanium dioxide can lose small, unweighable amounts of oxygen by interaction with radiant energy. This oxygen can easily recombine again as a part of a reversible photochemical reaction, particularly if there is no oxidizable material available. These small oxygen losses are important because they can cause significant changes in the optical and electrical properties of the pigment.Titanium dioxide should be stored in a well-closed container, protected from light, in a cool, dry place.
Forms and nomenclature
Titanium dioxide occurs in nature in three polymorphic crystal forms: anatase, rutile, and brookite. Moreover, under high pressure, the structure of all three polymorphs of titanium dioxide may be converted into that of α-PbO2. The following diagram summarises the main properties of these three polymorphisms: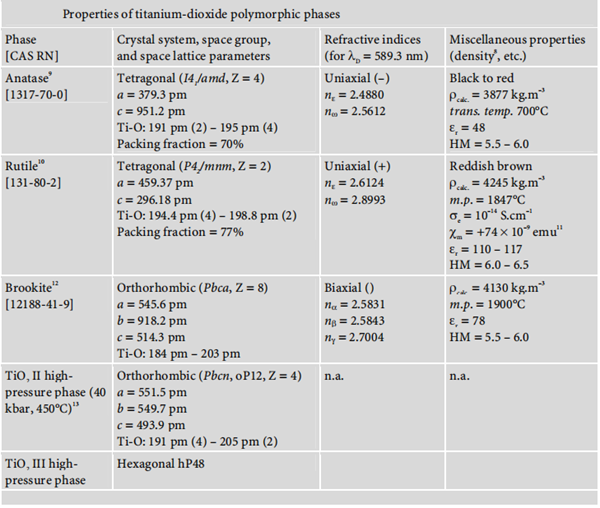
비 호환성
Titanium dioxide is incompatible with strong oxidizers and strong acids. Violent or incandescent reactions may occur with metals (e.g., aluminum, calcium, magnesium, potassium, sodium, zinc, and lithium).폐기물 처리
Land fill.Regulatory Status
Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (dental paste; intrauterine suppositories; ophthalmic preparations; oral capsules, suspensions, tablets; topical and transdermal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.이산화티타늄 준비 용품 및 원자재
원자재
준비 용품
이산화티타늄 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Dangtong Import and export Co LTD | +8615632927689 |
admin@hbdangtong.com | China | 991 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Hebei Dongdu Import and Export Co. LTD | +86-15333296769 +86-15333296769 |
manager@cndongdu.com | China | 71 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 |
catherine@yjchem.com.cn | China | 147 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 |
qinhe02@xaltbio.com | China | 1000 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 994 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7845 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8619133911216 |
fanfan@kangcang.com.cn | China | 340 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 |
sales03@chemcn.cn | China | 951 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 |
sarah@tnjone.com | China | 893 | 58 |