요오드트리메틸실란
|
|
요오드트리메틸실란 속성
- 녹는점
- <0°C
- 끓는 점
- 106 °C(lit.)
- 밀도
- 1.406 g/mL at 25 °C(lit.)
- 굴절률
- n
20/D 1.471(lit.)
- 인화점
- −25 °F
- 저장 조건
- -20°C
- 용해도
- 물과 반응
- 물리적 상태
- 액체
- 색상
- 무색~붉은색 투명
- Specific Gravity
- 1.47
- 수용성
- 물과 반응
- 감도
- Moisture & Light Sensitive
- Hydrolytic Sensitivity
- 8: reacts rapidly with moisture, water, protic solvents
- BRN
- 1731136
- 안정성
- Moisture Sensitive (Reactive)
- InChIKey
- CSRZQMIRAZTJOY-UHFFFAOYSA-N
- CAS 데이터베이스
- 16029-98-4(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-14-34 | ||
| 안전지침서 | 16-26-36/37/39-43-45-25 | ||
| 유엔번호(UN No.) | UN 2924 3/PG 2 | ||
| WGK 독일 | 3 | ||
| F 고인화성물질 | 8-21 | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | II | ||
| HS 번호 | 29310095 | ||
| 기존화학 물질 | KE-21044 |
요오드트리메틸실란 C화학적 특성, 용도, 생산
화학적 성질
Straw liquid주요 응용
Iodotrimethylsilane can be used as a versatile reagent for the mild dealkylation of ethers, carboxylic esters, lactones, carbamates, acetals, phosphonate and phosphate esters; cleavage of epoxides, cyclopropyl ketones; conversion of vinyl phosphates to vinyl iodides; neutral nucleophilic reagent for halogen exchange reactions, carbonyl and conjugate addition reactions; use as a trimethylsilylating agent for formation of enol ethers, silyl imino esters, and N-silylenamines, alkyl, alkenyl and alkynyl silanes; Lewis acid catalyst for acetal formation, α- alkoxymethylation of ketones, for reactions of acetals with silyl enol ethers and allylsilanes; reducing agent for epoxides, enediones, α-ketols, sulfoxides, and sulfonyl halides; dehydrating agent for oximes.제조 방법
Iodotrimethylsilane is prepared by mixing equimolar amounts of chlorotrimethylsilane and sodium iodide in acetonitrile, reduces various benzylic alcohols to the corresponding phenylalkanes. Other have been reported for the preparation of Trimethylsilyl Iodide(TMS-I): chlorotrimethylsilane undergoes halogen exchange with either lithium iodide in CHCl3 or sodium iodide in MeCN, which allows in situ reagent formation. Alternatively, hexamethyldisilane reacts with iodine at 25–61°C to afford TMS-I with no byproducts.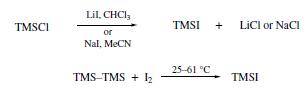
화학 반응
Trimethylsilyl iodide reacts under mild conditions in the absence of a catalyst with alkyl fluorides as well as with benzyl and tertiary alkyl chlorides and bromides to give good yields of alkyl iodides and the corresponding trimethylsilyl halides.일반 설명
Iodotrimethylsilane is a multipurpose reagent used in various organic reactions. It is used for the dealkylation of few compounds like lactones, ethers, acetals, and carbamates and trimethylsilylating agent for the synthesis of silyl imino esters, alkyl and alkenyl silanes, etc. It also acts as a Lewis acid catalyst and as a reducing agent in many organic reactions.Synthesis
This procedure describes a convenient method for the preparation of Iodotrimethylsilane: A 250-ml., two-necked, round-bottomed flask equipped with a magnetic stirring bar, an addition funnel for solids, and a reflux condenser bearing a nitrogen inlet is charged with 5.6 g. (0.21 mole) of aluminum powder and 16.2 g. (0.100 mole) of hexamethyldisiloxane and purged with nitrogen. The mixture is stirred and heated with an oil bath at 60°C as 50.8 g. (0.200 mole) of iodine is added slowly through the addition funnel over 55 minutes. The bath temperature is raised to ca. 140°C, and the mixture is heated under reflux for 1.5 hours. The reflux condenser is removed, and the flask is equipped for distillation at atmospheric pressure. The bath temperature is gradually raised from 140°C to 210°C, and the clear, colorless distillate is collected, yielding 32.6–35.3 g. (82–88%) of iodotrimethylsilane, b.p. 106–109°C.Purification Methods
Add a little antimony powder and fractionate with this powder in the still. 20 1.470. Stabilise the distillate with 1% wt of Cu powder. [Eaborn J Chem Soc 3077 1950, Beilstein 4 IV 4009.]요오드트리메틸실란 준비 용품 및 원자재
원자재
준비 용품
4-Fluorothiophenol
1-FLUORO-4-(TRIFLUOROMETHYLTHIO)BENZENE
(2S)-2-amino-3-(5-bromo-1H-indol-3-yl)propanoic acid
N,N-DiMethylMethyleneaMMoniuM Iodide
3-TRIMETHYLSILYL-2-PROPYN-1-OL
TRIMETHYLSILYLBENZENESULFONATE
5-Methyl-L-tryptophan
1-Cyclohexenyloxytrimethylsilane
1-(Trimethylsiloxy)cyclopentene
트리메틸페닐실란
사메틸 실란
요오드트리메틸실란 공급 업체
글로벌( 469)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| TCI AMERICA | 800-4238616 |
sales@tciamerica.com | Americas | 23662 | 75 |
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 |
daisy@crovellbio.com | China | 5964 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 |
admin@hbouhuang.com | China | 2259 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1807 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Win-Win Chemical CO., Limited | 0086-577-64498589 |
sales@win-winchemical.com | CHINA | 998 | 58 |