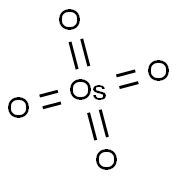
오스뮴산
|
|
오스뮴산 속성
- 녹는점
- 40°C
- 끓는 점
- 130 °C
- 밀도
- 1.04
- 증기 밀도
- 8.8 (vs air)
- 증기압
- 7 mmHg at 20 °C
- 인화점
- -40 °C
- 저장 조건
- 2-8°C
- 용해도
- 알코올, 에테르, 클로로포름, 벤젠, 수산화암모늄, 옥시염화인 및 사염화탄소에 용해됨
- 물리적 상태
- 액체
- Specific Gravity
- 5.10
- 색상
- 흰색에서 노란색, 또는 레몬색
- 냄새
- 2ppm(20mg/m3)에서 감지 가능한 매운 염소 같은 냄새
- 수용성
- 클로로포름, 알코올 및 에테르에 용해됩니다. 물, 유기 용매, 벤젠, 알코올, 에테르, 수산화 암모늄, 옥시염화인 및 사염화탄소에 용해됩니다.
- 감도
- Air Sensitive
- Merck
- 14,6893
- 안정성
- 안정적인. 강산, 염화수소, 유기 물질, 미세 분말 금속과 혼합되지 않습니다.
- CAS 데이터베이스
- 20816-12-0(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | O,T,C,T+,F,Xi,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 8-23/24/25-40-34-26/27/28-42/43-11-67-66-36/37/38-19-36/38-36/37 | ||
| 안전지침서 | 17-26-27-36/37/39-45-7/9-36/37-16-28-23 | ||
| 유엔번호(UN No.) | UN 3175 4.1/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | RN1140000 | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | I | ||
| HS 번호 | 28439090 | ||
| 유해 물질 데이터 | 20816-12-0(Hazardous Substances Data) | ||
| 독성 | LD50 oral (rat) 14 mg/kg LCLO inhal (rat) 40 ppm (4 h) PEL (OSHA) 0.0002 ppm (0.002 mg/m3) TLV-TWA (ACGIH) 0.0002 ppm (0.002 mg/m3) STEL (ACGIH) 0.0006 ppm (0.006 mg/m3) |
||
| IDLA | 1 mg Os/m3 | ||
| 기존화학 물질 | KE-27435 | ||
| 유해화학물질 필터링 | 97-1-125 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 사산화 오스뮴 및 이를 25% 이상 함유한 혼합물 |
오스뮴산 C화학적 특성, 용도, 생산
화학적 성질
Osmium tetroxide is a strong oxidant. Numerous organic substances reduce it to black osmium dioxide (OsO2) or to osmium metal.물리적 성질
Pale, yellow crystalline solid; chlorine-like acrid odor; monoclinic crystals having terahedral structure; density 5.1 g/cm3; melts at 40.6°C; vaporizes at 129.7°C; sublimation begins below its boiling point; vapor pressure 11 torr at 27°C; critical temperature 405°C; critical pressure 170 atm; moderately soluble in water, 7.24 g/100mL at 25°C; soluble in most organic solvents.용도
Osmium tetroxide is used in histopathological laboratories to stain the adipose tissue and as a stabilizing agent in scanning electron microscopy. In the chemical industry, it is used as a catalyst in the organic synthesis, particularly as the oxidizing agent in olefin-to-glycol conversion (546, 567). In the past, osmium tetroxide in the form of aqueous solution was used in forensic medicine to examine fingerprints. Osmium tetroxide is also used in medicine to treat rheumatoid arthritis.생산 방법
Osmium tetroxide is obtained by heating, at 300–400°C, finely divided osmium metal in the stream of air or oxygen (546). Commercially, it is received during osmium smelting and platinum annealing. Osmium tetroxide may also be produced by oxidizing osmium with aqua regia or nitric acid. It is often formed at room temperature from osmium metal powder.제조 방법
Osmium tetroxide is obtained as an intermediate during recovery of osmium metal from osmiridium or other noble metal minerals (See Osmium). In general, oxidation of an aqueous solution of an osmium salt or complex, such as sodium osmate with nitric acid, yields the volatile tetroxide which may be distilled out from the solution. In the laboratory, the compound can be prepared by oxidation of the osmium tetrachloride, OsCl4, or other halide solutions with sodium hypochlorite followed by distillation.Osmium tetroxide may also be produced by heating finely divided osmium metal in a stream of oxygen or air at 300 to 400°C.
정의
ChEBI: An osmium coordination entity consisting of four oxygen atoms bound to a central osmium atom via covalent double bonds.일반 설명
A colorless or yellow solid with a pungent odor of chlorine. Melting point about 104°F. Boiling point 266°F (begins to sublime below melting point). Density 4.9 g / cm3. Soluble in alcohol. Toxic by inhalation and a strong irritant to the eyes and mucous membranes.공기와 물의 반응
Soluble in water.반응 프로필
Osmium tetraoxide is incompatible with hydrochloric acid andeasily oxidized organic materials. Contact with other materials may cause fire. . Reacted explosively with1-methylimidazole [J. Chem. Soc., Dalton Trans., 1979, 1084].위험도
Osmium tetroxide is poisonous by all routes of exposure. The vapor is an eye irritant and can produce tears and damage. The vapor also can cause upper respiratory tract irritation.LD50 oral (mouse): 162 mg/kg
LCLO inhalation (mouse): 40 ppm (104 mg/m 3)/4 hr.
건강위험
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.화재위험
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.인화성 및 폭발성
NoncombustibleSafety Profile
Poison by ingestion, inhalation, and intraperitoneal routes. Human systemic effects by inhalation: lachrymation and other eye effects and structural or functional changes in trachea or bronch. Experimental reproductive effects. Mutation data reported. Explodes on contact with 1 -methylimidazole. Catalytic decomposition of hydrogen peroxide can be hazardous. See also OSMIUM잠재적 노출
Osmium may be alloyed with platinum metals, iron, cobalt, and nickel; and it forms compounds withtin and zinc. The alloy with iridium is used in the manufacture of fountain pen points, engraving tool; record player needles; electrical contacts; compass needles; fine machine bearings; and parts for watch and lock mechanisms. The metal is a catalyst in the synthesis of ammonia; and in the dehydrogenation of organic compounds. It is also used as a stain for histological examination of tissues. Osmium tetroxide is used as an oxidizing agent, catalyst, and as a fixative for tissues in electron microscopy. Other osmium compounds find use in photography. Osmium no longer is used in incandescent lights or in fingerprinting.저장
In particular, all work with osmium tetroxide should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Osmium tetroxide as solid or solutions should be stored in tightly sealed containers, and these should be placed in secondary containers.비 호환성
Osmium tetroxide is a strong oxidizer. Reacts with combustibles and reducing materials. Reacts with hydrochloric acid to form toxic chlorine gas. Forms unstable compounds with alkalis.폐기물 처리
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.오스뮴산 준비 용품 및 원자재
원자재
준비 용품
오스뮴산 관련 검색:
무수프탈산 무수프로피온산 무수트리멜리트산 디 -Tert- 부틸 디 카보네이트 말레산 무수물 크롬(6+) 트리산화물 숙신산무수물 오스뮴 tert-부틸알코올 오스뮴산 무수초산
BIS(PENTAMETHYLCYCLOPENTADIENYL)OSMIUM
OSMIUM (IV) OXIDE
OSMIUM TETRAOXIDE, 4 % IN WATER
OSMIUM TETRAOXIDE SOLID
OSMIUM TETRAOXIDE 1 GRAM AMPOULES
OSMIUM TETRAOXIDE, POLYMER-BOUND
OSMIUM TETRAOXIDE, 2 % IN WATER