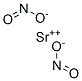아질산스트론튬
|
|
아질산스트론튬 속성
- 녹는점
- decomposes at 240℃ [HAW93]
- 밀도
- 2.800
- 용해도
- soluble in H2O
- 물리적 상태
- 흰색, 노란색 흡습성 바늘 모양의 결정
- 색상
- white-yellow hygroscopic needles
- 수용성
- g/100g, H2O: 43.1(35°C), 58.1(98°C); 고체상, Sr(NO2)2·H2O [KRU93]; 알코올에 불용성 [HAW93]
- CAS 데이터베이스
- 13470-06-9
안전
| 기존화학 물질 | KE-32236 | ||
|---|---|---|---|
| 유해화학물질 필터링 | 97-1-167 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 아질산 염류 및 이를 25% 이상 함유한 혼합물 |
아질산스트론튬 C화학적 특성, 용도, 생산
개요
Strontium nitrite has the formula of Sr(NO2)2 and the molecular weight of 179.6311 g/mol. It can be made by the usual double decomposition reaction of sodium nitrite and SrCl2. It forms a tetrahydrate which is isomorphous with the calcium and barium salts:Na2NO2 (aq) + SrCl2 ? SrNO2 (aq) + 2NaCl (aq)
If strontium nitrite is added to a mixture of magnesium– strontium nitrate, normally used in pyrotechnic formulations, it prevents aging of the nitrate mixture in the presence of water until usage in fireworks. Studies by isothermal microcalorimetry show that the addition of strontium nitrite to a 50% magnesium–50% strontium nitrate composition eliminated the induction reaction normally observed in closed ampoule studies in air at 50 C and relative humidity in the range 65–69%.
화학적 성질
white or yellowish powder(s); hygroscopic needles [HAW93]물리적 성질
Anhydrous strontium nitrite, Sr(NO2)2, has the CAS number of 13780-06-8 and occurs as yellow-white crystals. It is very hygroscopic. Its density is 2.23 g/cm3 and it decomposes at >650°C to form nitrogen oxides and SrO. It is slightly soluble in alcohol (0.04 g/100 ml at 20°C) but soluble in water at 72.1 g/100 ml at 20°C.Sr(NO2)2·4H2O loses two waters of hydration at about 80°C and one at 105°C to form the monohydrate. It loses the other at about 155°C. The anhydrate, if further heated, is unstable and oxidizes to the nitrate in air above 240°C:
Sr(NO2)2 + O2 + heat0Sr(NO3)2
Strontium nitrite monohydrate, Sr(NO2)2·H2O, has the CAS number of 13470-06-9 and a molecular weight of 197.6464 g/mol. It occurs as yellow-white crystals which are hygroscopic. Its density is 2.84 g/ cm3. In air, the crystals begin to decompose at about 245 °C. It is soluble in water at about 58.5 g/100 ml at 20 °C.
아질산스트론튬 준비 용품 및 원자재
원자재
준비 용품
아질산스트론튬 공급 업체
글로벌( 3)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 |
1026@dideu.com | China | 9214 | 58 |
| Hubei Enxing Biotechnology Co., Ltd | 16621771607 |
exbio_tech@163.com | China | 8369 | 58 |