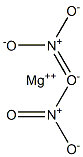질산마그네슘
|
|
질산마그네슘 속성
- 녹는점
- 648 °C(lit.)
- 끓는 점
- 1090 °C(lit.)
- 밀도
- 0.889 g/mL at 25 °C
- 증기압
- 1 mm Hg ( 621 °C)
- RTECS 번호
- OM3756000
- 인화점
- −26 °F
- 용해도
- H2O: 용해성
- 물리적 상태
- turnings
- 색상
- white cubic crystals, crystalline
- 수용성
- 물에 자유롭게 용해됩니다. 에탄올과 암모니아에 적당히 용해됩니다.
- 감도
- Hygroscopic
- Merck
- 13,5697
- 노출 한도
- ACGIH: TWA 2 ppm; STEL 4 ppm
OSHA: TWA 2 ppm(5 mg/m3)
NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3)
- CAS 데이터베이스
- 10377-60-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,Xi,O | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 34-36/37/38-8-36/38-35 | ||
| 안전지침서 | 43-7/8-26-17-36-45-36/37/39 | ||
| 유엔번호(UN No.) | UN 2056 3/PG 2 | ||
| WGK 독일 | - | ||
| TSCA | Yes | ||
| 위험 등급 | 5.1 | ||
| 유해 물질 데이터 | 10377-60-3(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-22725 |
질산마그네슘 C화학적 특성, 용도, 생산
개요
Magnesium nitrate has the molecular formula of Mg(NO3)2 and the molecular weight of 148.3152 g/ mol.Magnesium nitrate is prepared by the action of nitric acid on magnesium carbonate, oxide or hydroxide:
MgCO3 + 2HNO3 ? Mg(NO3)2 + CO2 +H2O
Mg(OH)2 + 2HNO3 ? Mg(NO3)2 + 2H2O
The salt crystallizing at room temperature after evaporation is the hexahydrate, Mg(NO3)2·6H2O. Two stable hydrates are formed, the hexahydrate [CAS number =13446-18-9] and the dihydrate, Mg(NO3)2· 2H2O [CAS number = 15750-45-5].
화학적 성질
Magnesium nitrate is white crystalline solid.용도
In pyrotechnics; in the concentration of nitric acid.제조 방법
The magnesium nitrate used in commerce has been synthesized in a variety of ways. The reaction between nitric acid and magnesium metal is one way and reaction with MgO is another. Magnesium hydroxide and ammonium nitrate also form the product but ammonia is released as a by-product:2HNO3 + Mg Mg(NO3)2 +H2
2HNO3 + MgO Mg(NO3)2 +H2O
Mg(OH)2 + 2NH4NO3 Mg(NO3)2 + 2NH3 + 2H2O
Since magnesium nitrate has a high affinity for water, heating the hexahydrate does not result in the dehydration of the salt. Instead, it decomposes into magnesium oxide, oxygen and nitrogen oxides:
4Mg(NO3)2·6H2O + heat 4MgO + 2NO2 + 2N2O + O2 + 6H2O
Heating the hexahydrate above its melting point first forms basic nitrates, such as Mg(NO3)2·4Mg(OH)2. It is this salt that decomposes at 400 C, forming magnesium oxide and oxides of nitrogen. The absorption of these nitrogen oxides in water is one possible way to synthesize HNO3. Although it is inefficient, it does not require the use of another strong acid and the mineral, nitromagnesite, can be used in this context.
정의
ChEBI: The inorganic nitrate salt of magnesium.일반 설명
A white crystalline solid. Produces toxic oxides of nitrogen if heated to decomposition. Used in pyrotechnics.공기와 물의 반응
Deliquescent. Water soluble.반응 프로필
Mixtures of Magnesium nitrate with alkyl esters may explode owing to the formation of alkyl nitrates; mixtures with phosphorus, tin(II) chloride, or other reducing agents may react explosively [Bretherick 1979 p. 108-109]. Noncombustible but will accelerate the burning of combustible materials. If large quantities are involved in a fire or the combustible material is finely divided an explosion may result. Prolonged exposure to fire or heat may result in an explosion. Magnesium nitrate has been reported to undergo spontaneous decomposition in dimethylformamide [Bretherick 5th ed., 1995]. Magnesium nitrate tends to behave as a strong oxidizer.위험도
Dangerous fire and explosion risk in contact with organic materials, strong oxidizing agent.건강위험
Exposure can cause mild irritation to the mucous membranes. Symptoms may include coughing and shortness of breath. Ingestion of large doses may cause dizziness, abdominal pain, vomiting, bloody diarrhea, weakness, convulsions, and collapse. Contact with skin may cause irritation, redness, and pain.Safety Profile
Probably a severe irritant to the eyes, skin, and mucous membranes. A powerful oxidizer. Violent decomposition on contact with dmethylformamide. When heated to decomposition it emits toxic fumes of NOx. See also NITRATES and MAGNESIUM COMPOUNDS.잠재적 노출
Magnesium nitrate is used in fireworks and in the production of concentrated nitric acid.운송 방법
UN1474 Magnesium nitrate, Hazard Class: 5.1; Labels: 5.1-Oxidizer비 호환성
A powerful oxidizer. Violent reaction with dimethylformamide, reducing agents; combustibles, fuels, organic and easily oxidizable matter질산마그네슘 준비 용품 및 원자재
원자재
준비 용품
4-(4-BROMOPHENYL)-3-PYRIDINECARBOXALDEHYDE
3-METHYL-2-PYRIDINECARBOXALDEHYDE
마그네슘 아세트산
methanation catalysts
2-HYDROXYMETHYL-3-METHYLPYRIDINE
naphtha steam reforming catalysts
마그네슘 니트레이트, 헥사수화물
5-BROMO-3-METHYL-2-PYRIDINECARBALDEHYDE
4-Methoxy-3-pyridinecarboxaldehyde
질산
6-(TRIFLUOROMETHYL)PYRIDINE-3-CARBOXALDEHYDE
4-(3-BROMOPHENYL)-3-PYRIDINECARBOXALDEHYDE
질산마그네슘 관련 검색:
스테아린산 마그네슘 황산마그네슘 황산마그네슘(7수화물) 질산 칼슘 질산 알루미늄 산화마그네슘 질산칼륨 염화 마그네슘 염화마그네슘 마그네슘 니트레이트, 헥사수화물 질산마그네슘 질산암모늄 마그네슘
Ammonium dihydrogen phosphate & Magnesium nitrate, Matrix Modifier Solution, Specpure|r
(Diisopropylamino) magnesium chloride
MAGNESIUM NITRATE, DODECAHYDRATE
Palladium nitrate & Magnesium nitrate, Matrix Modifier Solution, Specpure|r
CEROUS MAGNESIUM NITRATE