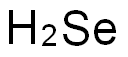셀레늄
|
|
셀레늄 속성
- 녹는점
- 217 °C (lit.)
- 끓는 점
- 684.9 °C (lit.)
- 밀도
- 4.81 g/mL at 25 °C (lit.)
- 증기압
- <1 Pa (20 °C)
- 저장 조건
- no restrictions.
- 용해도
- H2O: 용해성
- 물리적 상태
- 가루
- Specific Gravity
- 4.81
- 색상
- 흰색에서 크림색 흰색
- 비저항
- 1.2 μΩ-cm, 0°C
- 수용성
- 불용성
- Merck
- 13,8505
- 노출 한도
- TLV-TWA 0.2 mg(Se)/m3 (ACGIH, MSHA, and OSHA); IDLH 100 mg/m3.
- Dielectric constant
- 6.1(Ambient)
- 안정성
- 안정적인. 강산, 강산화제 및 대부분의 일반적인 금속과 호환되지 않습니다. 타기 쉬운.
- InChIKey
- SPVXKVOXSXTJOY-UHFFFAOYSA-N
- CAS 데이터베이스
- 7782-49-2(CAS DataBase Reference)
- IARC
- 3 (Vol. 9, Sup 7) 1987
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/38-53-33-23/25 | ||
| 안전지침서 | 26-61-45-28-20/21-28A | ||
| 유엔번호(UN No.) | UN 3440 6.1/PG 3 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | VS7700000 | ||
| TSCA | Yes | ||
| 위험 등급 | 9 | ||
| 포장분류 | III | ||
| HS 번호 | 28049090 | ||
| 유해 물질 데이터 | 7782-49-2(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 6700 mg/kg | ||
| IDLA | 1 mg Se/m3 | ||
| 기존화학 물질 | KE-30924 | ||
| 유해화학물질 필터링 | 97-1-134 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 셀레늄 또는 그 화합물과 셀렌화합물을 1% 이상 함유한 혼합물 |
셀레늄 C화학적 특성, 용도, 생산
개요
Selenium was discovered in 1817 by J?ns Jacob Berzelius. Especially noted was the similarity of the new element to the previously known tellurium. Selenium is an essential trace element atw0.1 ppm in diets. Selenium is a biologically active part of a number of important proteins, particularly enzymes involved in antioxidant defense mechanisms, thyroid hormone metabolism, and redox control of intracellular reactions. In humans and animals, selenium plays a role in protecting tissues from oxidative damage as a component of glutathione peroxidase.화학적 성질
Jewelers most frequently encounter selenium in the form of brass-black and gun-bluing compounds. Selenium print toner used by photographers is sometimes used by jewelers as a metal-coloring solution. These coloring mixtures usually contain selenic acid. Selenic acid can release hydrogen selenide gas that can cause illness, and used daily, it might enlarge the liver and spleen. Tellurium is sometimes used in association with selenium.물리적 성질
Selenium is a soft metalloid or semimetal that is similar to tellurium, located just belowit in the oxygen group, and sulfur, which is just above it in the same group. Selenium hasseveral allotropic forms that range from a gray metallic appearance to a red glassy appearance.These allotropic forms also have different properties of heat, conductivity, and density. In itsamorphous state, it is a red powder that turns black and becomes crystalline when heated.Crystalline selenium has a melting point of 220°C, a boiling point of 685°C, and a densityof 4.809 g/cm3.Isotopes
There are a total of 35 isotopes of selenium. Five of these are stable, anda sixth isotope has such a long half-life that it is also considered stable: Se-82 =0.83×10+20 years. This sixth isotope constitutes 8.73% of selenium’s abundance in theEarth’s crust, and the other five stable isotopes make up the rest of selenium’s abundanceon Earth.Origin of Name
Named for the Greek word selene, meaning “moon.” Jons Jacob Berzelius (1779–1848) discovered selenium and named it after the mineral called “eucairite,” which in Greek means “just in time.”출처
Selenium is the 67th most abundant element in Earth’s crust. It is widely spread over theEarth, but does not exist in large quantities. As a free element it is often found with the elementsulfur.There is only one mineral ore that contains selenium: eucairite (CuAgSe). Although rich inselenium, it is too scarce to be of commercial use. Almost all selenium is recovered from theprocessing of copper and the manufacturing of sulfuric acid as a leftover sludge by-product.This makes selenium’s recovery profitable. Recovering it from eucairite is not profitable.
Selenium is found in Mexico, Bosnia, Japan, and Canada. It can be found in recoverablequantities in some soils in many countries.
Characteristics
Crystalline selenium is a p-type semiconductor. It acts as a rectifier that can change electriccurrent from alternating current (AC) to direct current (to DC). It has photovoltaic proper ties, meaning it is able to convert light (radiant) energy that strikes it into electrical energy.Selenium’s resistance to the flow of electricity is influenced by the amount of light shining onit. The brighter the light, the better the electrical conductivity.Selenium burns with a blue flame that produces selenium dioxide (SeO2). Selenium willreact with most metals as well as with nonmetals, including the elements in the halogen group17.
용도
selenium is a trace mineral used for years in topical preparations for its anti-fungal properties. Selenium has been shown to have other protective effects such as repairing DnA, reducing the DnA-binding of carcinogens, and suppressing gene mutations. In laboratory studies, skin lotions containing selenium compounds have been shown to decrease uV-induced skin damage such as inflammation, blistering, and pigmentation.생산 방법
Selenium (Se), a nonmetallic element of the sulfur group, is widely distributed in nature. It is obtained along with tellurium as a by-product of metal ore refining, chiefly from copper deposits. About 16 ton is mined a year globally. The global refinery production of selenium, excluding the U.S. production, increased from about 1,400 metric ton in 2000 to about 1510 metric ton in 2008 and 1500 in 2009.Because selenium is present in fossil fuels, up to 90% of the selenium content in ambient air is emitted during their combustion. Air pollution concentrations averaged from 0.38 ng/m3 in remote areas to 13 ng/m3 in urban areas. The mass medium particle diameter was 0.92 mm. The worldwide emissions of 10,000 tons/year from natural sources exceed the atmospheric emissions from anthropogenic sources (5100 ton). However, 41,000 tons is emitted into the aquatic ecosystems. The largest contributors are electric power generating plants that produce 18,000 ton; manufacturing processes account for 12,000 ton.
Most of the world’s selenium today is provided by recovery from anode muds of electrolytic copper refineries. Selenium is recovered by roasting these muds with soda or sulfuric acid or by melting them with a soda and niter.
정의
selenium: Symbol Se. A metalloidelement belonging to group 16 (formerlyVIB) of the periodic table; a.n.34; r.a.m. 78.96; r.d. 4.81 (grey); m.p.217°C (grey); b.p. 684.9°C. There are anumber of allotropic forms, includinggrey, red, and black selenium. Itoccurs in sulphide ores of other metalsand is obtained as a by-product(e.g. from the anode sludge in electrolyticrefining). The element is asemiconductor; the grey allotrope islight-sensitive and is used in photocells,xerography, and similar applications.Chemically, it resemblessulphur, and forms compounds withselenium in the +2, +4, and +6 oxidation states. Selenium was discoveredin 1817 by J?ns Berzelius.일반 설명
Selenium is a reddish colored powder that may become black upon exposure to air. Selenium is toxic by ingestion. Selenium is used to manufacture electronic components and rubber.공기와 물의 반응
Insoluble in water.반응 프로필
SELENIUM, silicon, or sulfur ignites in fluorine gas at ordinary temperatures [Mellor 2:11-13 1946-47]. A mixture of barium carbide and selenium heated to 150° C becomes incandescence [Mellor 5:862 1946-47]. Calcium carbide and selenium vapor react with incandescence [Mellor 5:862 1946-47]. A moist mixture of selenium and chlorates, except the alkali chlorates, becomes incandescent. Selenium reacts violently with chromium trioxide [Mellor 11:233 1946-47]. Reaction of selenium and silver bromate (also potassium bromate) is violently explosive [Mellor 2, Supp1:763 1956]. Freshly reduced selenium reacts vigorously with nitric acid. Trace amounts of organic matter probably influenced the reaction [J. Chem. Soc. 1938 p.391]. The reaction between zinc and selenium or tellurium is accompanied by incandescence [Mellor 4:476-480 1946-47].위험도
The fumes and gases of most selenium compounds are very toxic when inhaled. SeO2 andSeS2 are toxic if ingested and very irritating to the skin. They are also carcinogenic.Although some compounds of selenium are poisonous, as an element it is essential in traceamounts for humans. It is recommended that 1.1 to 5 milligrams of selenium be included inthe daily diet. This amount can be maintained by eating seafood, egg yokes, chicken, milk,and whole grain cereals. Selenium assists vitamin E in preventing the breakdown of cells andsome chemicals in the human body.
화재위험
Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.Biotechnological Applications
Selenium is a component of a number of proteins. Selenium can exist as an anion at biological pH, which makes it able to both give and accept electrons. The best understood physiological functions of selenium are two enzyme functions. One of these functions is done as part of a family of proteins named glutathione peroxidase (one is found inside of cells, another is outside cells in places like the plasma).Glutathione peroxidase is part of the body's antioxidant defense network by eliminating peroxides, including hydrogen peroxide, which can be both precursors and products of free radicals. Selenium also functions in an enzyme that is part thyroid hormone synthesis. A more recently discovered selenium enzyme is known as thioredoxin reductase, which seems to have a number of regulatory roles within cells, and seems to affect antioxidant defense by inßuencing electron ßow in some reactions. One interesting point about this enzyme is that in rats, the enzyme activities can be increased by elevating selenium intake above those normally considered adequate.
Safety Profile
Poison by intravenous route. When heated to decomposition it emits toxic fumes of Se. See also SELENIUM and SELENIUM COMPOUNDS잠재적 노출
Most of the selenium produced is used in the manufacture of selenium rectifiers. It is also utilized as a pigment for ruby glass, paints, and dyes; as a vulcaniz- ing agent for rubber; a decolorizing agent for green glass; a chemical catalyst in the Kjeldahl test; as an insecticide; in the manufacture of electrodes, selenium photocells, sele- nium cells, and semiconductor fusion mixtures; in photo- graphic toning bathes; and for dehydrogenation of organic compounds. It is also used in veterinary medicine and in antidandruff shampoos. Se is used in radioactive scanning for the pancreas and for photostatic and X-ray xerography. It may be alloyed with stainless steel; copper, and cast steel. Selenium is a contaminant in most sulfide ores of copper, gold, nickel, and silver; and exposure may occur while removing selenium from these ores.운송 방법
UN3283 Selenium compound, solid, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous material, Technical Name Required.Purification Methods
Dissolve selenium in small portions in hot conc HNO3 (2mL/g), filter and evaporate to dryness to give selenious acid which is then dissolved in conc HCl. Pass SO2 gas through the solution whereby selenium (but not tellurium) precipitates. It is filtered off and washed with conc HCl. This purification process is repeated. The selenium is then converted twice to the selenocyanate by treating with a 10% excess of 3M aqueous KCN (CARE), heated for half an hour on a sand-bath and filtered. Add an equal weight of crushed ice to the cold solution, followed by an excess of cold, conc HCl, with stirring (in an efficient fume cupboard as HCN is evolved) which precipitates selenium powder. This is washed with water until colourless, and then with MeOH and is heated in an oven at 105o. Finally it is fused for 2hours in vacuo. It is cooled, crushed and stored in a desiccator [Tideswell & McCullough J Am Chem Soc 78 3036 1956].Structure and conformation
It takes three types of structures, metallic (grey) selenium, crystal (red) selenium, and amorphous selenium. The space lattice of metallic selenium belongs to the hexagonal system with two types, A and B. The B type is the most stable and the quasi-stable; A type changes to the B type slowly. The structure of the B type is an infinite zigzag chain containing three atoms in a unit cell with lattice constant of a=0.4355 nm, c=0.4949 nm, Se–Se=0.232 nm, and <Se– Se–Se=105° . The space lattice of crystal belongs to the monoclinic system with a=0.905 nm, b=0.907 nm, c=1.161 nm, Se–Se=0.234 nm, b=90841' , and <Se–Se–Se=105.38° ±2.3° . There may be two types for crystal selenium. Amorphous selenium changes to metallic selenium slowly at room temperature. Sometimes, selenium is classified into trigonal and amorphous types3 .비 호환성
Reacts violently with strong acids and strong oxidizers, chromium trioxide; potassium bromate;cadmium. Reacts with incandescence on gentle heating with phosphorous and metals, such as nickel, zinc, sodium, potassium, platinum. Reacts with water @ 50 ? C forming flammable hydrogen and selenious acids.폐기물 처리
Powdered selenium: dispose in a chemical waste landfill. When possible, recover selenium and return to suppliers주의 사항
During use and handling of selenium, occupational workers should be careful to avoid contact with the skin. Selenium compounds are considered very damaging to the liver, and hazardous.셀레늄 준비 용품 및 원자재
원자재
준비 용품
셀레늄 공급 업체
글로벌( 264)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 |
sales@zhuoerchem.com | China | 3015 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2931 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 |
1056@dideu.com | China | 3791 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 |
info@antaichem.com | CHINA | 9641 | 58 |
셀레늄 관련 검색:
3,4-다이하이드로-2,5,7,8-테트라메틸-2-(4,8,12-트라이메틸트 라이데실)-2H-벤조피란-6-올 나트륨 셀렌산염 이산화셀레늄 소듐 셀레나이트 나트륨 바이셀레나이트 셀레늄(IV) 플루오라이드 아셀렌산 이셀레늄화디페닐 셀레늄 메틸 옥시레인, 중합체 ,함유 옥시레인, 에테르 ,함유 (1,2-에탄딜- 디니트리로)테트라키스(프로판올)
5-Chlorovaleric acid
Pralmorelin
Diphenolic acid
4-(Diethylamino)salicylaldehyde
GALLIUM SELENIDE
SILVER SELENIDE
Molybdenum(IV) selenide
TUNGSTEN SELENIDE