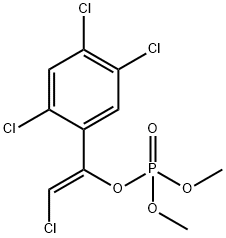
(Z)-2-클로로-1-(2,4,5-트리클로로페닐)비닐 디메틸 인산염
|
|
(Z)-2-클로로-1-(2,4,5-트리클로로페닐)비닐 디메틸 인산염 속성
- 녹는점
- 97-98 °C(lit.)
- 끓는 점
- 399.5±42.0 °C(Predicted)
- 밀도
- 1.520±0.06 g/cm3(Predicted)
- 증기압
- 6.0×10-6 Pa (20 °C)
- 저장 조건
- 0-6°C
- 용해도
- 아세토니트릴(약간), 메탄올(약간)에 용해됨
- 수용성
- 11mg l-1(20°C)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- Merck
- 13,9267
- CAS 데이터베이스
- 22248-79-9(CAS DataBase Reference)
- IARC
- 2B (Vol. 30, Sup 7, 112) 2017
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,N,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 21/22-50/53-36-20/21/22-11-22 | ||
| 안전지침서 | 36-60-61-36/37-16 | ||
| 유엔번호(UN No.) | UN 3077 9/PG 3 | ||
| RTECS 번호 | TB9100000 | ||
| HS 번호 | 29199000 | ||
| 유해 물질 데이터 | 22248-79-9(Hazardous Substances Data) | ||
| 독성 | LD50 in male, female rats (mg/kg): 1100, 1125 orally (Gaines) | ||
| 기존화학 물질 | KE-05920 |
| 그림문자(GHS): |
 
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Warning | |||||||||||||||||||||
| 유해·위험 문구: |
|
|||||||||||||||||||||
| 예방조치문구: |
|
(Z)-2-클로로-1-(2,4,5-트리클로로페닐)비닐 디메틸 인산염 C화학적 특성, 용도, 생산
개요
Tetrachlorvinphos was initially registered for use in the United States in 1966 for use on various food crops, livestock, and pet animals, and in around buildings. Its use on food crops were voluntarily canceled in the United States in 1987; however, it is used on food crops in developing countries. Tetrachlorvinphos is sold under the trade names Rabon and Gardona.화학적 성질
Technical tetrachlorvinphos is a tan-to-brown crystalline solid. Tetrachlorvinphos is stable at ,100 C and slowly hydrolyzed at 50°C. Aromatic odor.Soluble in water at 24°C 15 ppm; limited solubility in most aromatic hydrocarbons.
용도
Tetrachlorvinphos is used to control lepidopterous and dipterous larvae in fruit and lepidopterous larvae in cotton, maize, rice, tobacco and vegetables. It is also used against nuisance flies in animal houses, animal ectoparasites and stored product pests.위험도
Cholinesterase inhibitor. Questionable carcinogen.Carcinogenicity
When rats were given diets with 0, 4250, or 8500 ppm tetrachlorvinphos for 80 weeks, both males and females had a high incidence of thyroid C-cell hyperplasia, and females had increased incidences of adrenal cortical adenomas and thyroid C-cell adenomas .환경귀착
Tetrachlorvinphos is nonpersistent in the environment. The primary route of dissipation is through biotic degradation. Based on its use pattern, risks of contamination of groundwater or surface water by tetrachlorvinphos are minimal.신진 대사 경로
The chemical structure of tetrachlorvinphos is very close to that of chlorfenvinphos and the routes of metabolic breakdown have been shown to be very similar. Technical tetrachlorvinphos is usually >95% Z-isomer, unlike chlorfenvinphos which is an E/Z mixture. As with chlorfenvinphos, the major routes of detoxification are by dealkylation and hydrolysis to yield desmethyltetrachlorvinphos and 2,2’,4’,5’- tetrachloroacetophenone plus dimethyl phosphate, respectively. Further metabolism of the chloroacetophenone moiety then leads, via reduction or hydrolysis and glutathione-dependent displacement of the side chain chlorine substituent, to the formation of 1-(2,4,5-trichlophenyl) ethane-l,Z-diol and 1-(2,4,5-trichlorophenyl)ethan-l-owl hich are conjugated with glucose or glucuronic acid to afford the ultimate metabolites. Oxidation of the β carbon atom to give 2,4,5-trichloromandelic acid followed by decarboxylation leads to the formation of 2,4,5-trichlorobenzoic acid which is conjugated with glycine in some mammals as the final metabolite. The metabolic routes were summarised by Beynon et al. (1973).(Z)-2-클로로-1-(2,4,5-트리클로로페닐)비닐 디메틸 인산염 준비 용품 및 원자재
원자재
다이클로로아세틸 염화물
B-15
Aluminum paste
아조비스이소부티로니트릴
산소, 냉각된 액체
트리페닐 포스파이트
트라이클로로에틸렌
PENTACHLOROBENZENE
염화알루미늄
펜탄
메틸산 나트륨
삼염화 인
트리메틸포스파이트
메틸알콜
1,2,4-트라이클로로벤젠
a-클로로아세토페논
준비 용품
(Z)-2-클로로-1-(2,4,5-트리클로로페닐)비닐 디메틸 인산염 공급 업체
글로벌( 107)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 47465 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 19892 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; |
laboratory@coreychem.com | China | 30255 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 |
info@dycnchem.com | CHINA | 52867 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 |
peter68@ptchemgroup.com | China | 35453 | 58 |