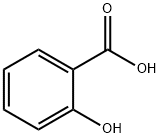
살리실산
|
|
살리실산 속성
- 녹는점
- 158-161 °C(lit.)
- 끓는 점
- 211 °C(lit.)
- 밀도
- 1.44
- 증기 밀도
- 4.8 (vs air)
- 증기압
- 1 mm Hg ( 114 °C)
- 굴절률
- 1,565
- 인화점
- 157 °C
- 저장 조건
- 2-8°C
- 용해도
- 에탄올: 1 M at 20°C, 투명, 무색
- 산도 계수 (pKa)
- 2.98(at 25℃)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 흰색에서 황백색까지
- pH 범위
- Non0 uorescence (2.5) to dark blue 0 uorescence (4.0)
- 수소이온지수(pH)
- 3.21(1 mM solution);2.57(10 mM solution);2.02(100 mM solution);
- 냄새
- 100.00%에서. 희미한 페놀성 열매
- ?? ??
- 견과류
- 수용성
- 1.8 g/L (20 ºC)
- 감도
- Light Sensitive
- 최대 파장(λmax)
- 210nm, 234nm, 303nm
- Merck
- 14,8332
- JECFA Number
- 958
- 승화점
- 70 ºC
- BRN
- 774890
- 안정성
- 안정적인. 피해야 할 물질로는 산화제, 강염기, 요오드, 불소 등이 있습니다. 타기 쉬운. 빛에 민감합니다.
- 주요 응용
- Semiconductors, nanoparticles, photoresists, lubricating oils, UV absorbers, adhesive, leather, cleaner, hair dye, soaps, cosmetics, pain medication, analgesics, antibacterial agent, treatment of dandruff, hyperpigmented skin, tinea pedis, onychomycosis, osteoporosis, beriberi, fungicidal skin disease, autoimmune disease
- InChIKey
- YGSDEFSMJLZEOE-UHFFFAOYSA-N
- LogP
- 2.01
- CAS 데이터베이스
- 69-72-7(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-41-36/37/38-36-20/21/22-11 | ||
| 안전지침서 | 26-39-37/39-36-36/37-16 | ||
| 유엔번호(UN No.) | UN 1648 3 / PGII | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | VO0525000 | ||
| 자연 발화 온도 | 500 °C | ||
| TSCA | Yes | ||
| HS 번호 | 29182100 | ||
| 유해 물질 데이터 | 69-72-7(Hazardous Substances Data) | ||
| 독성 | LD50 i.v. in mice: 500 mg/kg (Sota) | ||
| 기존화학 물질 | KE-20367 |
살리실산 C화학적 특성, 용도, 생산
개요
무색, 바늘모양의 결정이고 녹는점 159℃, 끓는점은 없고 승화한다. 물에 근소하게 녹아 산성을 나타낸다. 피부를 자극하고 약리작용이 있어 사마귀나 티눈을 뽑고 습진 치료에도 쓴다.용도
살리실산 또는 2-하이드록시 벤조산은 유기산의 하나로 유기합성에 많이 쓰인다. 식물 호르몬으로 작용하고, 의약품으로도 쓰인다.개요
Salicylic acid (from Latin salix, willow tree, from the bark of which the substance used to be obtained) is a mono hydroxy benzoic acid, a type of phenolic acid and a beta hydroxy acid. This colorless crystalline organic acid is widely used in organic synthesis and functions as a plant hormone. It is derived from the metabolism of salicin. In addition to being an important active metabolite of aspirin (acetylsalicylic acid), which acts in part as a prod rug to salicylic acid, it is probably best known for its use in anti-acne treatments. The salts and esters of salicylic acid are known as salicylates.화학적 성질
Salicylic acid has the formula C6H4(OH) COOH, where the OH group is ortho to the carboxyl group. It is also known as 2- hydroxybenzoic acid. It is poorly soluble in water (2 g / L at 20 °C). Aspirin (acetyl salicylic acid or ASA) can be prepared by the esterification of the phenolic hydroxyl group of salicylic acid with the acetyl group from acetic anhydride or acetyl chloride.물리적 성질
Salicylic acid. Appearance: white crystalline powder. Solubility: Absolutely soluble in ethanol, soluble in ether and chloroform, slightly soluble in water and anhydrous ether. Stability: Stable at room temperature, discomposes into phenol and carbon dioxide after rapidly heated. It’s partially acidic.Aspirin. Appearance: white crystal and decomposes at 136–140? °C. Melting point: 136?°C.?Aspirin is the acetyl derivative of salicylic acid with weak acidity. Its acidity coefficient is 3.5 at 25?°C. Stability: Aspirin decomposes rapidly in ammonium acetate, alkali metal of acetate, carbonate, citrate or hydroxide solutions. There are two crystal forms of aspirin including crystal form I and II.
출처
Unripe fruits and vegetables are natural sources of salicylic acid, particularly blackberries, blueberries, cantaloupes, dates, raisins, kiwi fruits, guavas, apricots, green pepper, olives, tomatoes, radish and chicory; also mushrooms. Some herbs and spices contain quite high amounts, although meat, poultry, fish, eggs and dairy products all have little to no salicylates. Of the legumes, seeds, nuts, and cereals, only almonds, water chestnuts and peanuts have significant amounts.역사
The Greek physician Hippocrates wrote in the 5th century BC about a bitter powder extracted from willow bark that could ease aches and pains and reduce fevers . This remedy was also mentioned in texts from ancient Sumer , Lebanon , and As syria .The active extract of the bark, called salicin, after the Latin name for the white willow (Salix alba), was isolated and named by the German chemist Johann Andreas Buchner in 1826. Raffaele Piria, an Italian chemist was able to convert the substance into a sugar and a second component, which on oxidation becomes salicylic acid.
Salicylic acid was also isolated from the herb meadowsweet (Filipendula ulmaria, formerly classified as Spiraea ulmaria) by German researchers in 1839. While their extract was somewhat effective, it also caused digestive problems such as gastric irritation, bleeding, diarrhea, and even death when consumed in high doses.
용도
Salicylic Acid is an Impurity of Acetylsalicylic Acid (A187780). Acetylsalicylic acid Impurity B.제조 방법
Prepared by heating sodium phenolate with carbon dioxide under pressure생산 방법
Salicylic acid is biosynthesized from the amino acid phenylalanine. In Arabidopsis thaliana it can also be synthesized via a phenylalanine - independent pathway.Sodium salicylate is commercially prepared by treating sodium phenolate ( the sodium salt of phenol ) with carbon dioxide at high pressure (100 atm ) and high temperature (390K) -a method known as the Kolbe-Schmitt reaction. Acidification of the product with sulfuric acid gives salicylic acid :
It can also be prepared by the hydrolysis of aspirin (acetylsalicylic acid) or methyl salicylate (oil of winter green) with a strong acid or base.
정의
ChEBI: A monohydroxybenzoic acid that is benzoic acid with a hydroxy group at the ortho position. It is obtained from the bark of the white willow and wintergreen leaves.Indications
Salicylic acid is a β-hydroxy acid that penetrates into the sebaceous gland and has comedolytic and anti-inflammatory properties. It can be used as an adjunctive therapy and is found in cleansers, toners, masks, and peels.Salicylic acid is keratolytic and at concentrations between 3% and 6% causes softening of the horny layers and shedding of scales. It produces this desquamation by solubilizing the intercellular cement and enhances the shedding of corneocytes by decreasing cell-to-cell cohesion. In concentrations >6%, it can be destructive to tissue. Application of large amounts of the higher concentration of salicylic acid can also result in systemic toxicity. Salicylic acid is used in the treatment of superficial fungal infections, acne, psoriasis, seborrheic dermatitis, warts, and other scaling dermatoses. When it is combined with sulfur, some believe that a synergistic keratolytic effect is produced. Common preparations include a 3% and 6% ointment with equal concentration of sulfur; 6% propylene glycol solution (Keralyt); 5% to 20% with equal parts of lactic acid in flexible collodion for warts (Duofilm, Occlusal); in a cream base at any concentration for keratolytic effects; as a 60% ointment for plantar warts; and in a 40% plaster on velvet cloth for the treatment of calluses and warts (40% salicylic acid plaster).
일반 설명
Odorless white to light tan solid. Sinks and mixes slowly with water.공기와 물의 반응
Sublimes and forms vapor or dust that may explode [USCG, 1999].위험도
Respiratory alkalosis, hyperkalemia, hyperthermia, dehydration, convulsions, shock, res- piratory paralysis, respiratory acidosis, lesions and death from respiratory collapse; fetotoxic.건강위험
Inhalation of dust irritates nose and throat. Vomiting may occur spontaneously if large amounts are swallowed. Contact with eyes causes irritation, marked pain, and corneal injury which should heal. Prolonged or repeated skin contact may cause marked irritation or even a mild burn.Mechanism of action
Salicylic acid has been shown to work through several different pathways. It produces its anti - inflammatory effects via suppressing the activity of cyclo oxygenase (COX), an enzyme which is responsible for the production of pro - inflammatory mediators such as the prostaglandins. Notably, it does this not by direct inhibition of COX, unlike most other non-steroidal anti-inflammatory drugs (NSAIDs), but instead by suppression of the expression of the enzyme (via a yet-un elucidated mechanism) . Salicylic acid has also been shown to activate adenosine monophosphate-activated protein kinase (AMPK), and it is thought that this action may play a role in the anticancer effects of the compound and its prod rugs aspirin and salsalate. In addition, the anti diabetic effects of salicylic acid are likely mediated by AMPK activation primarily through allosteric conformational change that increases levels of phosphorylation. Salicylic acid also uncouples oxidative phosphorylation which leads to increased ADP:ATP and AMP:ATP ratios in the cell. Consequently, salicylic acid may alter AMPK activity and subsequently exert its anti-diabetic properties through altered energy status of the cell. Even in AMPK knock - out mice, however, there is an anti-diabetic effect demonstrating that there is at least one additional, yet - unidentified action of the compound.Pharmacology
Aspirin is a nonsteroidal anti-inflammatory drug (NSAID). The main pharmacological effect is to inhibit prostaglandin metabolism and thromboxane synthesis by inhibiting prostaglandin metabolism-required cyclooxygenase, via irreversible acetylation of 530 serine residues in the hydroxyl of COX-1 polypeptide chain, which results in COX-1 inactivation, blocks the conversion of arachidonic acid into thromboxane A2 pathway and then inhibits the platelet aggregation.Prostaglandin is a hormone produced locally in the body. It can pass the pain to the brain, regulate body temperature in the hypothalamus and cause inflammation. Inhibition of prostaglandin synthesis can have antipyretic, analgesic, antiinflammatory and antirheumatic effects. The adverse effects of aspirin are mainly gastrointestinal symptoms such as nausea, vomiting, upper abdominal discomfort or pain. It can also cause allergic reactions, cardiotoxicity, liver and kidney damage and Wright’s syndrome. In addition, high doses of aspirin can cause salicylic acid reactions such as headache, dizziness, tinnitus, hearing loss and other central nervous system symptoms.
Clinical Use
The clinical application of aspirin varies with the therapeutic dose. Low-dose aspirin (75–300?mg/day) has antiplatelet aggregation effect and can be used to prevent and treat the coronary and cerebrovascular thrombosis and other postoperative thrombosis. The middle dose of aspirin (0.5–3? g/day) has antipyretic analgesic effects, so it is commonly used in the treatment of fever, headache, toothache, neuralgia, muscle pain and menstrual pain. High doses of aspirin (more than 4?g/day) have anti-inflammatory and antirheumatic effects for the treatment of acute rheumatic fever and rheumatoid arthritis. In addition, aspirin is used for the treatment of skin and mucous membrane lymphadenopathy (Kawasaki disease) in paediatric.부작용
Salicylic acid's side effects include erythema and scaling.Safety Profile
Poison by ingestion, intravenous, and intraperitoneal routes. Moderately toxic by subcutaneous route. An experimental teratogen. Human systemic effects by skin contact: ear tinnitus. Mutation data reported. A skin and severe eye irritant. Experimental reproductive effects. Incompatible with iron salts, spirit nitrous ether, lead acetate, iodine. Used in the manufacture of aspirin. When heated to decomposition it emits acrid smoke and irritating fumes.잠재적 노출
Used as a topical keratolytic agent; in manufacture of aspirin, salicylates, resins, as a dyestuff intermediate; prevulcanization inhibitor; analytical reagent; fungicide, antiseptic, and food preservative.Purification Methods
It has been purified by steam distillation, by recrystallisation from H2O (solubility is 0.22% at room temperature and 6.7% at 100o), absolute MeOH, or cyclohexane and by sublimation in a vacuum at 76o. The acid chloride (needles) has m 19-19.5o, b 92o/15mm, the amide has m 133o (yellow needles from H2O), the O-acetyl derivative has m 135o (rapid heating and the liquid resolidifies at 118o), and the O-benzoyl derivative has m 132o (aqueous EtOH). [IR: Hales et al. J Chem Soc 3145 1954, Bergmann et al. J Chem Soc 2351 1950]. [Beilstein 10 IV 125.]비 호환성
iron salts; lead acetate; iodine. Forms an explosive mixture in air.살리실산 준비 용품 및 원자재
원자재
준비 용품
와파린
알루미논
술포살리실 산
Acid Black 168
Solvent Red BL
SALICYLIC ACID 4-OCTYLPHENYL ESTER
살리실산 나트륨
5-아미노셀릭일 산
Ethyl 2-hydroxybenzoate
흑색 194 염료
Direct Dark Brown NM
4-(2-(5-CHOLRO-2-METHOXY BENZAMIDO)ETHYL)BENZENESULFONYL CHLORIDE
OLSALAZINE
Isocarbophos
3,5- 디아이오도살리실산
Acid Orange 88
guacetisal
Neutral Black 2S-RL
살리실산벤질
Sieber Linker
ISOFENPHOS-METHYL
Acid Red 215
5-CHLORO-2-METHOXYBENZOYL CHLORIDE
Acid violet 68
아스피린
아밀 살리실산염
3,5-Dibromosalicylic acid
Acid Black 60
Acid Black 172
Mordant Yellow 10
5-포밀살리실산
Isobutyl salicylate
5-Chloro-2-hydroxybenzoic acid
살리실산이소아밀
흑색 107 염료
Solvent Red KL
페닐 살리실산
3,5-Dichlorosalicylic acid
Neutral Brown Rl
Acid violet 90
살리실산 공급 업체
글로벌( 1331)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Xi'an ZB Biotech Co.,Ltd | +8618591943808 |
sales01@xazbbio.com | China | 816 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| ENBRIDGE PHARMTECH CO., LTD. | +8613812269233 |
tinayang@enbridgepharm.com | China | 303 | 58 |
| Shandong Yanshuo Chemical Co., Ltd. | +86-18678179670 +86-18615116763 |
sales@yanshuochem.com | China | 101 | 58 |
| Shandong Zhishang New Material Co., Ltd. | +8617653113209 |
sales002@sdzschem.com | China | 3050 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 |
sales@myuchem.com | China | 6710 | 58 |
| Wuhan senwayer century chemical Co.,Ltd | +undefined-27-86652399 +undefined13627115097 |
market02@senwayer.com | China | 874 | 58 |
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 |
daisy@crovellbio.com | China | 5964 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 |
kyra@quanjinci.com | China | 1532 | 58 |
| Changzhou Rokechem Technology Co., Ltd. | 18758118018 |
sales001@rokechem.com | China | 255 | 58 |