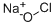차아염소산나트륨
|
|
차아염소산나트륨 속성
- 녹는점
- -16 °C
- 끓는 점
- 111 °C
- 밀도
- 1.25 g/mL at 20 °C
- 증기압
- 17.5 mmHg ( 20 °C)
- 굴절률
- 1.3870
- 저장 조건
- 2-8°C
- 용해도
- 메탄올(약간 용해됨), 물(약간 용해됨)
- 물리적 상태
- 액체
- 색상
- 연노랑
- Specific Gravity
- 1.209
- 냄새
- 연한 녹색에서 연한 노란색의 액체, 염소계 표백제 냄새
- 수용성
- 분해된다.
- Merck
- 14,8628
- 노출 한도
- ACGIH: Ceiling 2 mg/m3
OSHA: TWA 2 mg/m3
NIOSH: IDLH 10 mg/m3; Ceiling 2 mg/m3
- Dielectric constant
- 6.7(Ambient)
- BCS Class
- 1
- 안정성
- 안정적인. 산과 접촉하면 유독가스(염소)가 방출됩니다. 빛에 민감합니다. 강산, 아민, 암모니아, 암모늄염, 환원제, 금속, 아지리딘, 메탄올, 포름산, 페닐아세토니트릴과 호환되지 않습니다.
- LogP
- -3.42 at 20℃
- CAS 데이터베이스
- 7681-52-9(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,Xi,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 31-34-36/38-36/37/38-50 | ||
| 안전지침서 | 26-36/37/39-45-50A-28A-36-61-50-28 | ||
| 유엔번호(UN No.) | UN 1791 8/PG 3 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | NH3486300 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | III | ||
| HS 번호 | 28289000 | ||
| 유해 물질 데이터 | 7681-52-9(Hazardous Substances Data) | ||
| 독성 | Skin contact with the solid hypochlorite pentahydrate or its concentrated solution can cause irritation. Ingestion may cause corrosion of mucous membranes and gastric perforation. | ||
| 기존화학 물질 | KE-31506 |
차아염소산나트륨 C화학적 특성, 용도, 생산
물성
성 상 이 품목은 무엷은 녹황색 액체 또는 분말로서 염소의 냄새를 가지고 있다.화학 반응
산소계 표백제나 산성 물질과 반응하여 맹독성인 염소 기체가 발생한다.화학 반응
예를 들어 염산과 섞으면2HCl + NaOCl → NaCl + H2O + Cl2↑
용도
화학적으로 굉장히 산화력이 강하기 때문에 표백작용과 살균작용이 있으며 보통 흰 의류를 표백하거나 화장실 청소할 때 주로 쓰이고 그 외에도.독성
염소 가스를 흡입하게되면 호흡기 내의 수분과 염소가 반응하여 염산이 생성되며, 이는 폐와 기관지를 포함한 호흡기 전반에 큰 피해를 입힌다. 즉 염산이 폐포와 기관지, 인후두 등을 녹여버린다!! 게다가 눈의 망막까지 손상시킨다! 그래서 염소 가스는 제1차 세계 대전 중 제2차 이프르 전투에서 독일군이 독가스로 사용했다. 이에 장기간 노출되면 사망할 수 있다.확인시험
(1) 이 품목은 염색반응시험법에 따라 시험할 때, 황색을 나타낸다.
(2) 이 품목에 묽은염산을 가하면 가스가 발생한다.
(3) 이 품목에 적색리트머스지를 넣으면 리트머스지는 청색으로 변하고 다음에는 퇴색한다.
정량법
이 품목 3g을 정밀히 달아 이에 물 50mL을 가하고 요오드칼륨 2g 및 초산(1→4) 10mL을 가한 후 바로 밀봉하여 암소에 15분간 방치하고, 유리된 요오드를 0.1N 치오황산나트륨용액으로 적정한다(지시약 : 전분시액). 따로 같은 방법으로 공시험을 한다. 다만, 차아염소산나트륨수 제조장치를 통하여 제조되는 차아염소산나트륨수는 10mL을 정확히 취하여 이에 물 50mL을 가하고 요오드칼륨 1g 및 초산(1→4) 5mL을 가하여 유리된 요오드를 0.01N 치오황산나트륨용액으로 적정한다. 종말점은 액의 청색이 사라질 때로 한다.
정의
정 의 이 품목은 차아염소산나트륨을 주성분으로 하는 것을 말하며, 식염수를 전기분해의 방법으로 얻어지는 것도 포함한다.
화학적 성질
Sodium hypochlorite, NaOCl, is an air-unstable,pale green crystalline solid that is soluble in cold water, decomposes in hot water, and has a sweet aroma. It generally is available in one of two strengths. The household liquid bleach contains about 5.25 wt% NaCIO. The commercial product(sometimes called 15% bleach) contains 150g/L available chlorine. This is equivalent to about 13 wt% sodium hypochlorite. Sodium hypochlorite is used as a bleaching agent for paper pulp and textiles, as an oxidizing reagent, as a disinfectant, as a chemical intermediate,and in medicines.The hypochlorite ion (OCI-) is similar to wet chlorine gas in its effects on materials. Not many metals exhibit good resistance even at low temperatures and concentrations. Because hypochlorite solutions are unstable at neutral and lower pHs,they normally contain excess alkali,which modifies the aggressiveness somewhat.
물리적 성질
Anhydrous sodium hypochlorite explodes; the pentahydrate is a pale-green crystalline solid; orthorhombic structure; density 1.6 g/cm3; melts at 18°C; decomposed by CO2 in the air; soluble in water, 29.3 g/100 mL at 0°C; the aqueous solution is highly stable.역사
Sodium hypochlorite exists as an aqueous solution from 5 15% NaOCl and is commonly called bleach. Household bleach is typically a 5.25% solution, and industrial bleach is sold as a 12% solution. When sodium hypochlorite is used in this entry, it is assumed to be the aqueous solution, which is clear, slightly yellow, corrosive, and has a distinctive chlorine smell. Chorine gas was discovered by Carl Wilhelm Scheele (1742 1786) in 1774 and known initially as depholgisticated salt spirit. In 1787, the French chemist Claude Louis Berthollet (1749 1822) experimented with aqueous solution of chlorine gas as bleaching agents. Based on Berthollet's work, the Javel Company located on the outskirts of Paris began to produce bleaches in 1788. Chlorine gas was dissolved in a solution of soda potash (potassium carbonate) to obtain a product called liqueur de Javel, which was potassium hypochlorite. Potash treated with chlorine gas was also used to produce bleaching powders. In 1820, Antoine Germaine Labarraque (1777 1850), an apothecary, substituted cheaper soda ash (sodium carbonate) for potash to produce Eau de Labarraque or Labarraque solution, which was sodium hypochlorite. Eau de Labarraque was used as a disinfectant and to bleach paper. Bleaching powders, borax, lye, and blueing were used as bleaches throughout the 19th century.Sodium hypochlorite is the primary hypochlorite used as a bleach and disinfectant, accounting for 83% of world hypochlorite use, with calcium hypochlorite accounting for the remaining 17%. Approximately 1 million tons of sodium hypochlorite was used globally in 2005, with about half this amount used in households for laundry bleaching and disinfection. The other half was used primarily for wastewater and drinking water treatment; other uses include pool sanitation, bleaching of pulp, paper, and textiles, and as an industrial chemical.
용도
Sodium hypochlorite is marketed only as an aqueous solution because the anhydrous solid is highly unstable and can explode. The solid pentahydrate also is unstable in air, decomposed by reaction with carbon dioxide from air. Aqueous solutions are very stable. They are used for bleaching textiles and paper pulp; in cleaning solutions; in water purification; as a disinfectant for swimming pools; and as a germicide and topical antiinfective. The hypochlorite also is used as an oxidizing agent in many preparative reactions. It is an ingredient of commercial bleaching products such as Clorox and Dazzle.제조 방법
Sodium hypochlorite solution is obtained by passing chlorine into sodium hydroxide solution. The pentahydrate is obtained by crystallization.정의
ChEBI: An inorganic sodium salt in which hypochlorite is the counterion.일반 설명
Green to yellow watery liquid with an odor of bleaching liquid odor. Sinks and mixes with water.공기와 물의 반응
Water soluble. Decomposes into chlorine and oxygen gases in hot water.반응 프로필
Salts of hypochlorous acid, HClO. Generally toxic, irritants and powerful oxidizers, particularly in the presence of water at higher temperature as they decompose to release oxygen and chlorine gases. On contact with urea they form the highly explosive NCl3 . When heated or on contact with acids, they produce highly toxic fumes of chlorine gas [Sax, 9th ed., 1996, p. 1905]. Can react with sulfuric acid to produce heat and chlorine gas.위험도
Fire risk in contact with organic materials. Toxic by ingestion, strong irritant to tissue.건강위험
Liquid can be irritating to skin and eyes if contact is maintained.화재위험
Behavior in Fire: May decompose, generating irritating chlorine gas.부작용
Sodium hypochlorite, commonly known as bleach, may be used as a disinfectant solution. It is a strong irritant; however, isolated reports of CoU to sodium hypochlorite exist. The mechanism for the Cou is uncertain.Hostynek et al. describe a 36-year-old woman who developed an intensely pruritic maculopapular rash to a hypochlorite-containing cleaning product that she spilled on her leg. The rash progressed to involve her trunk and extremities and was associated with teary eyes, dyspnea, and facial edema. There was a history of a previous sensitizing event, and open testing to 1% sodium hypochlorite produced an immediate urticarial reaction. The authors suggest that this could be due to an immunological mechanism given the generalized symptoms; however, no confirmatory testing was performed and the potential of sodium hypochlorite to cause nonimmunologic Cou was evident with four of 10 controls experiencing a wheal-and-flare reaction to open application of 6% sodium hypochlorite.
Caliskan et al. described a 32-year-old female who developed severe lip edema and breathing difficulty after using a sodium hypochlorite irrigation during endodontic treatment. A scratch test to sodium hypochlorite resulted in immediate erythema and edema that began to extend up the patient’s arm. She also had a history of breathing difficulties and had developed dermatitis from her hands to elbows with the use of household cleaning agents.
Neering reported on a patient who had experienced intermittent Cou to chlorinated pools and contact with a cleansing agent containing sodium hypochlorite. A scratch test to chlorinated water was strongly positive in this patient, but negative in five controls, and closed patch testing to sodium hypochlorite was strongly positive at three hours.
Safety Profile
Mddly toxic by ingestion. Human systemic effects by ingestion: somnolence, blood pressure lowering, corrosive to skin, nausea or vomiting. Human mutation data reported. An eye irritant. Corrosive and irritating by ingestion and inhalation. The anhydrous salt is highly explosive and sensitive to heat or friction. Explosive reaction with formic acid (at So), phenylacetonitrile. Reacts to form explosive products with amines, ammonium salts (e.g., ammonium acetate, (NH4)2CO3, ammonium nitrate, ammonium oxalate, (NH4)3P04), aziridme, methanol. Violent reaction with phenyl acetonitrile, cellulose, ethyleneimine. Solutions in water are storage hazards due to oxygen evolution. When heated to decomposition it emits toxic fumes of NaaO and Cl-. Used as a bleach.차아염소산나트륨 준비 용품 및 원자재
원자재
준비 용품
3,3-Dimethyl-2-oxobutyric acid
4-CHLORO-1H-PYRAZOLE-3-CARBOXYLIC ACID
3-CHLORO-6-NITRO (1H)INDAZOLE
chlorinated sodium phosphate
안스라니릭산에틸에스테르
6-AMINO-3-CHLORO (1H)INDAZOLE
IMMEDIAL BRILLIANT GREEN GB
4,6-DIMETHYL-PYRIMIDINE-2-SULFONYL FLUORIDE
1-Amino-2,4-dibromoanthraquinone
4-Chloroimidazole
2-(4-모르폴리노)에탄설폰산
1,3-디클로로-5,5-디메틸 하이단토인
Magentagreencrystals
Direct Fast Yellow RR
벤즈 [CD] 인돌 -2- (1H) - 온
2,5-Dichloronicotinic acid
DIRECT YELLOW 29
5-CHLOROPYRIDIN-2(1H)-ONE
루테늄(III) 염화물
1-Chlorobenzotrizole
4-METHYL-PYRIMIDINE-2-SULFONYL FLUORIDE
N-옥시디에틸렌-2-벤조티아조릴 설펜아미드
아트라진
MEQUINDOX
3,3-Dimethylglutaric acid
3-METHYLTHIOPHENE-2-CARBONYL CHLORIDE
요오드포름
4-CHLORO-3-METHYL-1H-PYRAZOLE
8-히드록시-7-요오드-퀴노린술폰산
2-CHLOROSULFONYL-PYRIDINIUM, CHLORIDE
Vat Black 25
C.I.Vat Red 29
3,5-DICHLORO-2-PYRIDONE
5-METHYL-PYRIMIDINE-2-SULFONYL FLUORIDE
1-피렌카르복시산
1,3-다이브로모-5,5-다이메틸하이단토인
Vat Orange 11
3,5,6-TRICHLORO-PYRIDINE-2-CARBOXYLIC ACID
N-사이클로헥셀-벤조티아조릴 설펜아미드
Vat Brown 1
차아염소산나트륨 공급 업체
글로벌( 616)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 |
bj1@bj-chem.com | China | 298 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9352 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1011 | 58 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2931 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |