노르말헥산
|
|
노르말헥산 속성
- 녹는점
- -95 °C
- 끓는 점
- 68.95 °C(lit.)
- 밀도
- 0.659 g/mL at 25 °C(lit.)
- 증기 밀도
- 3.5 (vs air)
- 증기압
- 40 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.388
- 인화점
- 30 °F
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- 에탄올, 에틸 에테르 및 클로로포름에 매우 잘 녹습니다.
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- >14 (Schwarzenbach et al., 1993)
- 색상
- 무색의
- Specific Gravity
- 0.660 (20/4℃)
- 냄새
- 65~248ppm에서 약한 휘발유 냄새가 감지됩니다.
- 상대극성
- 0.009
- 폭발한계
- 1.0-8.1%(V)
- Odor Threshold
- 1.5ppm
- 수용성
- 불용성
- 최대 파장(λmax)
- λ: 200 nm Amax: ≤0.70
λ: 225 nm Amax: ≤0.10
λ: 250 nm Amax: ≤0.01
- Merck
- 14,4694
- BRN
- 1730733
- Henry's Law Constant
- 0.238, 0.413, 0.883, 0.768, and 1.56 at 10, 15, 20, 25, and 30 °C, respectively (EPICS, Ashworth et al., 1988)
- Dielectric constant
- 2.0(-90℃)
- 노출 한도
- TLV-TWA 50 ppm (~175 mg/m3) (ACGIH), 500 ppm (~1750 mg/m3) (OSHA); IDLH 5000 ppm (NIOSH).
- 안정성
- 안정적인. 산화제, 염소, 불소, 과염소산마그네슘과 호환되지 않습니다. 가연성이 높습니다. 공기와 쉽게 폭발성 혼합물을 형성합니다. 낮은 인화점을 참고하세요.
- InChIKey
- VLKZOEOYAKHREP-UHFFFAOYSA-N
- LogP
- 4 at 20℃ and pH7
- CAS 데이터베이스
- 110-54-3(CAS DataBase Reference)
- NIST
- Hexane(110-54-3)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,Xn,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-38-50/53-65-67-62-51/53-48/20-36/37/38 | ||
| 안전지침서 | 9-16-29-33-60-61-62-36/37-45-36/37/39-53-26 | ||
| 유엔번호(UN No.) | UN 3295 3/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | MN9275000 | ||
| F 고인화성물질 | 3-10 | ||
| 자연 발화 온도 | 225 °C | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | II | ||
| HS 번호 | 29011000 | ||
| 유해 물질 데이터 | 110-54-3(Hazardous Substances Data) | ||
| 독성 | LC50 (4 hr) in mice by inhalation: 48000 ppm; LD50 orally in rats: 32.0 g/kg (Couri, Milks) | ||
| IDLA | 1,100 ppm [10% LEL] | ||
| 기존화학 물질 | KE-18626 |
노르말헥산 C화학적 특성, 용도, 생산
물성
헥 산 휘발유 처럼 냄새를 가진 무색 액체 이다. 안정적인 합니다. 에이전트, 염소, 불 소, 마그네슘 과염소산염을 산화와 함께 호환 되지 않습니다. 가연성. 쉽게 공기를 가진 폭발성 혼합물을 형성 한다. 참고 낮은 플래시 포인트.용도
1.유기 합성;2.입니다. 용 매가 식물 기름 추출;
3.추출 에이전트, 화학 시 약.
포장, 보관 및 운송
페인트 드럼 또는 유조선 포장으로 밀봉. 스토리지 건조 하 고 차가운 장소에 배치 되어야 합니다, 그리고 창 고 가수분해를 방지 하기 위해 물과의 접촉을 피하기 위해 환기 시설이 있어야 합니다. 교통도로 철도에 위험한 화학 물질의 수송에 중화 인민 공화국의 규정을 준수 해야순도시험
(1) 비중 : 이 품목의 비중은 0.665~0.687이어야 한다.
(2) 굴절률 : 이 품목의 굴절률 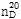 은 1.374~1.386이어야 한다.
은 1.374~1.386이어야 한다.
(3) 황화합물 : 이 품목 5mL에 질산은암모니아시액 5mL를 가하여 잘 흔들어 섞으면서 광선을 피하여 60℃에서 5분간 가열할 때, 갈색을 나타내어서는 아니 된다.
(4) 황산정색물 : 이 품목 5mL를 네슬러관에 취하여 94.5~95.5% 황산 5mL를 가하여 5분간 심하게 진탕 혼합할 때, 황산층의 색은 비색표준용액 B보다 진하여서는 아니 된다.
(5) 벤젠 : 이 품목 50mL를 취해 여기에 내부표준물질용액 50mL를 가해서 혼합하고 이것을 시험용액으로 한다. 따로 벤젠표준용액 50mL를 취하여 내부표준물질용액 50mL를 가하여 혼합하고 이것을 표준용액으로 한다. 시험용액 및 표준용액을 다음의 조건에서 가스크로마토그래피를 행할 때, 시험용액의 표시피크높이(H)와 내부표준물질의 표시피크높이(Hs)와의 비 H/Hs는 표준용액의 표시피크높이(H'')와 내부표준물질의 표시피크높이(Hs'')와의 비 H''/Hs'' 보다 크지 않아야 한다. 다만, 내부표준물질용액은 메틸이소부틸케톤 0.5mL를 취해 n-헥산(자외부흡수스펙트럼측정용)을 가해서 100mL로 한 것을, 벤젠표준용액은 벤젠 0.05mL를 취해 n-헥산(자외부흡수스펙트럼측정용)을 가해서 100mL로 한 것을 사용한다.
조작조건
칼 럼 : 내경 3~4mm, 길이 2~3m의 스텐레스관 또는 유리관
칼럼충전제 : 177~250μm 가스크로마토그래피용 담체에 대해서 10%되는 양의 폴리에틸렌글리콜 6,000을 함유하는 클로로포름용액을 가해 클로로포름을 증발하고 건조한 것
칼럼온도 : 50~70℃의 일정온도
검 출 기 : 수소염이온화검출기(FID)
캐리어가스 및 유량 : 질소가스를 이용한다. 벤젠이 약 5분에 나오도록 캐리어가스의 유량을 조정한다.
(6) 증류시험 : 이 품목은 비점 및 유분측정법중 제2법에 따라 유분을 측정할 때, 64~70℃에서 95% 이상을 유출하여야 한다.
(7) 증발잔류물 : 이 품목 150mL를 주의하면서 수욕상에서 가열하여 증발한 다음 105℃에서 30분간 건조할 때, 그 잔류물은 2mg 이하이어야 한다.
(8) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(9) 다환방향족탄화수소 : 이 품목 25mL를 취하여 125mL 분액깔대기에 옮기고 n-헥산 25mL를 가하여 잘 흔들어 섞은 다음 디메틸설폭시드 5mL를 가하여 1분간 세게 흔들어 섞고 층이 분리될 때까지 정치한다. 하층을 분액깔대기에 옮기고 n-헥산 2mL를 가하여 2분간 세게 흔들어 섞은 후 층이 분리될 때까지 정치한 다음 하층을 취하여 시험용액으로 한다. 디메틸설폭시드 5mL 및 헥산 25mL를 각각 취하여 1분간 흔들어 섞고 정치한 후 얻어진 하층을 공시험용액으로 하여 파장 260~420nm에서 시험용액의 흡광도를 측정한다. 따로, 대조액으로 나프탈렌 7.0mg을 정밀히 달아 이소옥탄 1,000mL에 녹인 액을 이소옥탄을 공시험용액으로 하여 파장 275nm에서 대조액의 흡광도를 측정한다. 파장 260~420nm에서 시험용액의 흡광도를 측정할 때, 그 값은 파장 275nm에서 측정한 대조액의 흡광도 값의 1/3을 초과하여서는 아니 된다.
정의
이 품목은 석유 성분중에서 n-헥산의 비점부근에서 증류하여 얻어진 것이다.
화학적 성질
n-Hexane is a highly flammable liquid, usually isolated from crude oil, and has extensive industrial applications as a solvent in adhesive bandage factories and other industries.
It is highly toxic, triggering several adverse health effects, i.e., nausea, skin irritation, dizziness, numbness of limbs, CNS depression, vertigo, and respiratory tract irritation to animals and humans. Occupational exposure of industrial workers has demonstrated motor polyneuropathy. Workers associated with long-term glue sniffi ng showed adverse effects in the form of degeneration of axons and nerve terminals.
물리적 성질
Clear, colorless, very flammable liquid with a faint, gasoline-like odor. An odor threshold concentration of 1.5 ppmv was reported by Nagata and Takeuchi (1990).용도
n-Hexane is a chief constituent of petroleumether, gasoline, and rubber solvent. It is usedas a solvent for adhesives, vegetable oils,and in organic analysis, and for denaturingalcohol.정의
ChEBI: An unbranched alkane containing six carbon atoms.일반 설명
Clear colorless liquids with a petroleum-like odor. Flash points -9°F. Less dense than water and insoluble in water. Vapors heavier than air. Used as a solvent, paint thinner, and chemical reaction medium.공기와 물의 반응
Highly flammable. Insoluble in water.반응 프로필
HEXANE may be sensitive to light. Hexane may also be sensitive to prolonged exposure to heat. Hexane can react vigorously with oxidizing materials. This would include compounds such as liquid chlorine, concentrated O2, sodium hypochlorite and calcium hypochlorite. Hexane is also incompatible with dinitrogen tetraoxide. Hexane will attack some forms of plastics, rubber and coatings. .위험도
Flammable, dangerous fire risk.건강위험
n-Hexane is a respiratory tract irritant andat high concentrations a narcotic. Its acutetoxicity is greater than that of n-pentane.Exposure to a concentration of 40,000 ppmfor an hour caused convulsions and death inmice. In humans a 10-minute exposure toabout 5000 ppm may produce hallucination,distorted vision, headache, dizziness, nausea,and irritation of eyes and throat. Chronicexposure to n-hexane may cause polyneuritis.The metabolites of n-hexane injected inguinea pigs were reported as 2,5- hexanedioneand 5-hydroxy-2-hexanone, which arealso metabolites of methyl butyl ketone(DiVincenzo et al. 1976). Thus methyl butylketone and n- hexane should have similartoxicities. The neurotoxic metabolite, 2,5-hexanedione, however, is produced considerablyless in n-hexane. However, in the caseof hexane, the neurotoxic metabolite 2,5-hexanedione is produced to a much lesserextent. Continuous exposure to 250 ppmn-hexane produced neurotoxic effects in animals. Occupational exposure to 500 ppmmay cause polyneuropathy (ACGIH 1986).
Inhalation of n-hexane vapors have shownreproductive effects in rats and mice.
인화성 및 폭발성
Hexane is extremely flammable (NFPA rating = 3), and its vapor can travel a considerable distance to an ignition source and "flash back." Hexane vapor forms explosive mixtures with air at concentrations of 1.1 to 7.5 % (by volume).Hydrocarbons of significantly higher molecular weight have correspondingly higher vapor pressures and therefore present a reduced flammability hazard. Carbon dioxide or dry chemical extinguishers should be used for hexane fires.
화학 반응
Reactivity with Water: No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.잠재적 노출
n-Hexane is industrial chemical, emul sifier, in manufacture of plastics, resins; as a solvent, par ticularly in the extraction of edible fats and oils; as a laboratory reagent; and as the liquid in low temperature thermometers. Technical and commercial grades consist of 45 85% hexane, as well as cyclopentanes, isohexane, and 1% to 6% benzene.Carcinogenicity
Male rabbits exposed to 3000 ppm hexane (8 h/day, 6 days/week for 24 weeks) developed papillary proliferation of nonciliated bronchiolar cells. No tumors were found in mice painted with hexane and croton oil as cocarcinogen, presumably for the lifetime of each animal. Hexane is inactive as a tumorpromoting agent.환경귀착
Biological. Hexane may biodegrade in two ways. The first is the formation of hexyl hydroperoxide, which decomposes to 1-hexanol followed by oxidation to hexanoic acid. The other pathway involves dehydrogenation to 1-hexene, which may react with water giving 1-hexanol (Dugan, 1972). Microorganisms can oxidize alkanes under aerobic conditions (Singer and Finnerty, 1984). The most common degradative pathway involves the oxidation of the terminal methyl group forming 1-hexanol. The alcohol may undergo a series of dehydrogenation steps forming a hexanal followed by oxidation to form hexanoic acid. The fatty acid may then be metabolized by β-oxidation to form the mineralization products, carbon dioxide and water (Singer and Finnerty, 1984).Photolytic. An aqueous solution irradiated by UV light at 50 °C for 1 d resulted in a 50.51% yield of carbon dioxide (Knoevenagel and Himmelreich, 1976). Synthetic air containing gaseous nitrous acid and exposed to artificial sunlight (λ = 300–450 nm) photooxidized hexane into two isomers of hexyl nitrate and peroxyacetal nitrate (Cox et al., 1980).
Chemical/Physical. Complete combustion in air yields carbon dioxide and water vapor.
저장
hexane should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers.운송 방법
UN1208 Hexanes, Hazard Class: 3; Labels: 3-Flammable liquid.Purification Methods
Purify as for n-heptane. Modifications include the use of chlorosulfonic acid or 35% fuming H2SO4 instead of conc H2SO4 in washing the alkane, and final drying and distilling from sodium hydride. Unsaturated impurities can be removed by shaking the hexane with nitrating acid (58% H2SO4, 25% conc HNO3, 17% water, or 50% HNO3, 50% H2SO4), then washing the hydrocarbon layer with conc H2SO4, followed by H2O, drying, and distilling over sodium or n-butyl lithium. It can also be purified by distillation under nitrogen from sodium benzophenone ketyl solubilised with tetraglyme. Also purify it by passage through a silica gel column followed by distillation [Kajii et al. J Phys Chem 91 2791 1987]. It is a FLAMMABLE liquid and a possible nerve toxin. [Beilstein 1 IV 338.] Rapid purification: Distil, discarding the first forerun and stored over 4A molecular sieves.비 호환성
May form explosive mixture with air. Contact with strong oxidizers may cause fire and explo sions. Contact with dinitrogen tetraoxide may explode @ 28℃.Attacks some plastics, rubber and coatings. May accumulate static electrical charges, and may cause ignition of its vapors.폐기물 처리
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.노르말헥산 준비 용품 및 원자재
원자재
준비 용품
노르말헥산 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shandong Yanshuo Chemical Co., Ltd. | +86-18678179670 +86-18615116763 |
sales@yanshuochem.com | China | 101 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 |
peter68@ptchemgroup.com | China | 35453 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 |
qinhe02@xaltbio.com | China | 1000 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 |
bj1@bj-chem.com | China | 298 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32480 | 60 |