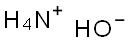암모니아수
|
|
암모니아수 속성
- 녹는점
- -77°C
- 끓는 점
- 36°C
- 밀도
- 0.91 g/mL at 20 °C
- 증기 밀도
- 1.2 (vs air)
- 증기압
- 115 mmHg at 20 °C for 29% solution
- 저장 조건
- 2-8°C
- 용해도
- 물 에 용해
- 물리적 상태
- 액체, 단일 하위 끓는점 석영 증류
- 산도 계수 (pKa)
- 9.3(at 25℃)
- 색상
- 무색의
- Specific Gravity
- approximate 0.96 (10%, 15℃)
- 수소이온지수(pH)
- 10.09(1 mM solution);10.61(10 mM solution);11.12(100 mM solution);
- 냄새
- 17ppm에서 감지 가능한 강한 자극성 암모니아 냄새
- 폭발한계
- 27%
- 수용성
- 물과 섞일 수 있습니다.
- 최대 파장(λmax)
- λ: 260 nm Amax: 0.01
λ: 280 nm Amax: 0.01
- Merck
- 14,494
- BRN
- 3587154
- 안정성
- 안정적인. 구리, 구리 합금, 산, 아연 도금 철, 아연, 알루미늄, 청동, 디메틸 황산염, 수은, 알칼리 금속과 호환되지 않습니다.
- InChIKey
- VHUUQVKOLVNVRT-UHFFFAOYSA-N
- CAS 데이터베이스
- 1336-21-6(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 34-50-22 | ||
| 안전지침서 | 26-36/37/39-45-61 | ||
| 유엔번호(UN No.) | UN 2672 8/PG 3 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | BQ9625000 | ||
| F 고인화성물질 | 34 | ||
| 자연 발화 온도 | 690 °C (for ammonia) | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | III | ||
| HS 번호 | 28142000 | ||
| 유해 물질 데이터 | 1336-21-6(Hazardous Substances Data) | ||
| 독성 | LD50 oral (rat) 350 mg/kg PEL (OSHA) 35 ppm (27 mg/m3) TLV-TWA (ACGIH) 25 ppm (17 mg/m3) STEL (ACGIH) 35 ppm (27 mg/m3) |
||
| 기존화학 물질 | KE-01688 | ||
| 유해화학물질 필터링 | 97-1-184 | ||
| 사고대비 물질 필터링 | 44 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 암모니아 및 이를 10% 이상 함유한 혼합물 |
암모니아수 C화학적 특성, 용도, 생산
순도시험
(1) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 2.0ppm 이하이어야 한다.
(2) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(3) 증발잔류물 : 이 품목 11mL(약 10g에 해당되는 양)을 미리 항량시킨 백금제 또는 자제접시에서 증발시킨 다음 105℃에서 1시간 건조할 때, 그 잔류량은 0.02% 이하이어야 한다.
(4) 산화되기 쉬운 물질 : 이 품목 4mL에 물 6mL를 가하고 묽은황산 약간 과량과 0.1N 과망간산칼륨용액 0.1mL를 가할 때, 엷은 홍색이 10분 이내에 없어져서는 아니 된다 .
확인시험
이 품목에 염산을 적신 유리막대를 가까이 할 때, 진한 흰 연기가 발생한다.
정량법
미리 1N 황산 35mL를 넣고 마개를 한 다음 무게를 단 공전플라스크에 10℃이하로 미리 냉각한 이 품목을 가능한 한 바닥부분에서 10mL 눈금이 있는 피펫을 사용하여 취한다. 피펫 외벽에 묻어 있는 액을 닦아내고 처음 용액은 버리고 2mL를 플라스크에 가한다. 이 때 최소한 1mL는 피펫에 남아 있어야 한다. 플라스크를 마개로 막고 혼합한 다음 검체채취량을 구하기 위해 다시 무게를 달고 과잉의 산을 1N 수산화나트륨용액으로 적정한다(지시약 : 메틸레드시액 1~2방울).
1N 황산 1mL = 17.03mg NH3
개요
Ammonium hydroxide is a colorless, liquid solution with a characteristic and pungent odor. It is ammonia combined with water. Ammonia (NH3) is a compound consisting of nitrogen and hydrogen. Both ammonia and ammonium hydroxide are very common compounds, found naturally in the environment (in air, water, and soil) and in all plants and animals, including humans. Ammonia is a source of nitrogen, an essential element for plants and animals. Ammonia is also produced by the human body – by our organs and tissues and by beneficial bacteria living in our intestines.Ammonia plays an important role in protein synthesis in the human body. In brief summary, all living things need proteins, which are comprised of some 20 different amino acids. While plants and microorganisms can synthesize most amino acids from the nitrogen in the atmosphere, animals cannot. For humans, some amino acids cannot be synthesized at all and must be consumed as intact amino acids. Other amino acids, however, can be synthesized by microorganisms in the gastrointestinal tract with the help of ammonia ions. Thus, ammonia is a key player in the nitrogen cycle and in protein synthesis. Ammonia also helps maintain the body's pH balance.
화학적 성질
Ammonium hydroxide exists only in the form of an aqueous solution. The compound is prepared by dissolving NH3 in H2O and usually is referred to in industrial trade as aqua ammonia. For industrial procurements, the concentration of NH3 in solution is normally specified in terms of the specific gravity (degrees Baum′e, °Be). Common concentrations are 20 °Be and 26 °Be. The former is equivalent to a sp gr of 0.933, or a concentration of about 17.8% NH3 in solution; the latter is equivalent to a sp gr of 0.897, or a concentration of about 29.4% NH3. These figures apply at a temperature of 60 °F (15.6 °C). Reagent grade NH4OH usually contains approximately 58% NH4OH (from 28 to 30% NH3 in solution).용도
Ammonium hydroxide is used as a cleaning agent and sanitizer in many household and industrial cleaners. Ammonium hydroxide is also used in the manufacture of products such as fertilizer, plastic, rayon and rubber. Aqueous ammonia is corrosive to aluminum alloys, copper, copper alloys, and galvanized surfaces. Aqueous ammonia is an excellent acid neutralizer.정의
Ammonium hydroxide,NH40H, is a hydrate of anunonia and exists in crystalline form at -79°C. Normally, it is only found in an aqueous solution also known as aquaanunonia and anunonia water. It is prepared by dissolving NH3 inH20. Reagent grade anunonium hydroxide contains from 28 to 30% NH3 at 15.6 °C. Industrial sales specify the concentration of NH3 in solution in terms of specific gravity. Common concentrations are 20 °Be, which would bea concentration of 17.8% NH3 (specific gravity 0.933) and 26 °Be (specific gravity 0.897), or a concentration of 29.4% NH3. Ammonium hydroxide is an excellent medium for the reaction of NH3 (which becomes the NH4 radical in solution) with other compounds for the preparation of anunonium salts and other nitrogen-containing chemicals. It is an ingredientin deodorants, etching compounds, and cleaning and bleaching materials. Ammoniumhydroxide, as aqua ammonia, finds wide use as a neutralizing agent,because it is inexpensive and strongly alkaline.일반 설명
Ammonium hydroxide is a colorless aqueous solution. Concentration of ammonia ranges up to approximately 30%. Ammonia vapors (which arise from the solution) irritate the eyes.공기와 물의 반응
Water soluble. Generates a small amount of heat when diluted with water.반응 프로필
Ammonium hydroxide reacts exothermically with acids. Evolves toxic gaseous ammonia with strong bases. Reacts extremely violently with dimethyl sulfate [NFPA 491M 1991]. Reacts with aqueous silver nitrate sodium hydroxide to give a black precipitate of silver nitride. Such a precipitate can explode on stirring [MCA Case History 1554 1968]. Aqueous ammonia and Hg react to form an explosive solid, likely a fulminate. (Thodos, G. Amer. Inst. Chen. Engrs. J., 1964, 10, 274.).위험도
Liquid and vapor extremely irritating, especially to eyes.건강위험
Ammonium hydroxide solutions are alkaline solutions, meaning they have high pH level. As a result, ammonium hydroxide is a severe eye, skin, and respiratory tract irritant, and readily burns tissue with which it comes in contact. Splashes to the eye may be serious, as contact may cause severe burns, irritation pain and possibly blindness. Direct contact with skin may cause severe burns if the chemical is not quickly rinsed away with copious amounts of water. Inhaling mists of ammonium hydroxide may result in irritation of the nose and throat with symptoms including burning, coughing, choking and pain. Inhaling concentrated mist may result in pulmonary edema and shock. Ingesting ammonium hydroxide may cause pain and burns of the esophagus and gastrointestinal tract.TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
화재위험
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.인화성 및 폭발성
Ammonia vapor is slightly flammable (NFPA rating = 1) and ignites only with difficulty. Ammonia forms explosive mixtures with air in the range 16 to 25%. Water, carbon dioxide, or dry chemical extinguishers should be used for ammonia fires.농업용
Ammonium hydroxide is also known as ammonia solution, aqua ammonia, aqueous ammonia or ammonia liquor. It is the solution of ammonia in water and is commonly referred to as ammonium hydroxide. It is the simplest nitrogen solution made by forcing compressed ammonia (anhydrous ammonia) gas into water.Safety Profile
A human poison by ingestion. An experimental poison by inhalation and ingestion. A severe eye irritant. Human systemic irritant effects by ocular and inhalation routes. Mutation data reported. Incompatible with acrolein, nitromethane, acrylic acid, chlorosulfonic acid, dimethyl sulfate, halogens, (Au + aqua regia), HCl, HF, HNO3, oleum, ppropiolactone, propylene oxide, AgNO3, Ag2O, (Ag20 + C2H5OH), AgMn04, H2SO4. Dangerous; liquid can inflict burns. Use with adequate ventilation. When heated to decomposition it emits NH3 and NO2.잠재적 노출
It is used in detergents, stain removers, bleaches, dyes, fibers, and resins.저장
All work with this substance should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact.Containers should be tightly sealed to prevent escape of vapor and should be stored in a cool area separate from halogens, acids, and oxidizers. Containers stored in warm locations may build up dangerous internal pressures of ammonia gas.
운송 방법
UN2672 Ammonia solutions, relative density between 0.880 and 0.957 at 15 C in water, with .10% but not .35% ammonia, Hazard class: 8; Labels: 8-Corrosive material.비 호환성
Solution is strongly alkaline. Violent reaction with strong oxidizers, acids (exothermic reaction with strong mineral acids). Shock-sensitive compounds may be formed with halogens, mercury oxide; silver oxide. Fire and explosions may be caused by contact with β-propiolactone, silver nitrate; ethyl alcoho; silver permanganate; trimethylammonium amide; 1-chloro-2,4-dinitrobenzene, o-chloronitrobenzene, platinum, trioxygen difluoride; selenium difluoride dioxide; boron halides; mercury, chlorine, iodine; bromine, hypochlorites, chlorine bleach; amides, organic anhydrides; isocyanates, vinyl acetate; alkylene oxides; epichlorohydrin; aldehydes. Attacks some coatings, plastics and rubber. Attacks copper, brass, bronze, aluminum, steel, zinc, and their alloys.폐기물 처리
Dilute with water, neutralize with HCl and discharge to sewer.암모니아수 준비 용품 및 원자재
원자재
준비 용품
cytochrome C solution
네오마이신 황산
2-Amino-3,6,8-naphthalenetrisulfonic acid
N,1,3-Trimethyl-1H-pyrazole-5-carboxamide ,97%
암모늄옥살산 제2철
2-ANTHRACENECARBOXYLIC ACID
(1-METHYL-1H-BENZIMIDAZOL-2-YL)METHYLAMINE
1,2-benzisothiazol-3(2H)-one 1,1-dioxide, ammonium salt
2-AMINO-3-PYRIDINECARBOXALDEHYDE HCL
2,4-Dimethylimidazole
2,6-DIMETHYL-3-HYDROXYPYRIDINE
FOSAMINE AMMONIUM
글루포신에이트-암모늄
Ethyl 3-amino-4,4,4-trifluorocrotonate
aluminium oxide sol
메토카밤올
6-Chloropyridazin-3-amine
Hydrofining catalyst FH-5
adhesive for electrostatic flocking EX-1
N,1,5-Trimethyl-1H-pyrazole-3-carboxamide ,97%
Isepamicine
2,4-Dichlorobenzonitrile
Doxycycline monohydrate
4-Nitrobenzenesulfonamide
2-CHLORO-6-METHYLPYRIMIDIN-4-AMINE
polyalumium sulfate chloride
(6-BROMO-2-PYRIDINYL)-CARBAMIC ACID,1,1-DIMETHYLETHYL ESTER
1,3-DIMETHYL-1H-PYRAZOLE-5-CARBOXAMIDE
DL-Cystine
2,4,5-TRIMETHYL-3-OXAZOLINE
L-glutamic acid monoammonium
AMMONIUM PHOSPHOMOLYBDATE
synthetic thickener KG-201
Direct Blue 199
테트라사이아노금산(III) 칼륨
emulsifier SOPE-15
에핀에피린
1,5-Dimethyl-1H-pyrazole-3-carboxamide ,97%
5-methylquinolin-8-ol
6-BROMONAPHTHALEN-2-AMINE
암모니아수 공급 업체
글로벌( 724)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7377 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9352 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 47465 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 |
info@antaichem.com | CHINA | 9641 | 58 |