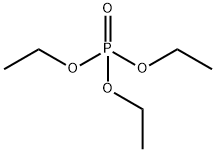
트리에틸인산염
|
|
트리에틸인산염 속성
- 녹는점
- -56 °C
- 끓는 점
- 215 °C (lit.)
- 밀도
- 1.072 g/mL at 25 °C (lit.)
- 증기 밀도
- 6.28 (vs air)
- 증기압
- 1 mm Hg ( 40 °C)
- 굴절률
- n
20/D 1.403(lit.)
- 인화점
- 240 °F
- 저장 조건
- Store below +30°C.
- 용해도
- 500g/l(느린 분해)
- 물리적 상태
- 액체
- Specific Gravity
- 1.072
- 색상
- 투명한
- 수소이온지수(pH)
- 7 (H2O, 20℃)
- 냄새
- 순한 사과주
- 폭발한계
- 1.2-10%(V)
- 수용성
- 녹는
- Hydrolytic Sensitivity
- 7: reacts slowly with moisture/water
- Merck
- 14,9674
- BRN
- 1705772
- Dielectric constant
- 7.2(20℃)
- 안정성
- 안정적인. 타기 쉬운. 강한 산화제, 물과 호환되지 않습니다.
- InChIKey
- DQWPFSLDHJDLRL-UHFFFAOYSA-N
- LogP
- 1.11 at 20℃
- CAS 데이터베이스
- 78-40-0(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-36 | ||
| 안전지침서 | 25-26 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | TC7900000 | ||
| 자연 발화 온도 | 845 °F | ||
| TSCA | Yes | ||
| HS 번호 | 29190090 | ||
| 유해 물질 데이터 | 78-40-0(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 1165 mg/kg | ||
| 기존화학 물질 | KE-28646 |
트리에틸인산염 C화학적 특성, 용도, 생산
화학적 성질
Triethyl Phosphate (TEP) is a colorless, high-boiling liquid and containing 17 wt % phosphorus; mild odor. Very stable at ordinary temperatures, compatible with many gums and resins, soluble in most organic solvents, miscible with water. When mixed with water is quite stable at room temperature, but at elevated temperatures it hydrolyzes slowly.
TEP is useful as a solvent in many applications, as a plasticizer for tough, fire-resistant plastics, and as an agricultural chemical as an intermediate in preparing tetraethyl pyrophosphate (TEPP).
Like other phosphate esters, Triethyl phosphate damages nerves and is a cholinesterase inhibitor. It is regarded as moderately toxic. Two other alkyl phosphates with toxicities probably similar to that of triethylphosphate are tributylphosphate, (n-C4H9O)3PO, and tris(2-ethylhexyl)-phosphate, (C8H17O)3PO.
용도
Triethyl phosphate is use as a flame retardant in the manufacture of polyisocyanurate (PIR) and polyurethane (PUR) foam insulation and thermoset plastic products. The chemical compound is also used as a viscosity reducer in plastic resins, and as a catalyst, solvent or intermediate in the production of pesticides, pharmaceuticals, lacquers and other products.As ethylating agent; formation of polyesters which are used as insecticides.생산 방법
Triethyl phosphate is manufactured from diethyl ether and phosphorus pentoxide via a metaphosphate intermediate.Prepared by the reaction of tetraethyl hypophosphate with ethanol in the presence of aluminum ethoxide or by treating triethyl phosphate with diethyl hydrogen phosphate.
정의
ChEBI: Triethyl phosphate is a trialkyl phosphate that is the triethy ester derivative of phosphoric acid. It derives from an ethanol.일반 설명
Triethyl phosphate [78-40-0] is a colorless, corrosive liquid. Combustible. Slowly dissolves in water and sinks in water. Severely irritates skin, eyes and mucous membranes. It is manufactured from diethyl ether and phosphorus pentoxide via a metaphosphate intermediate. Triethyl phosphate has been used commercially as an additive for polyester laminates and in cellulosics. In polyester resins it functions as a viscosity depressant and as a flame retardant. The viscosity-depressant effect of triethyl phosphate in polyester resin permits high loadings of alumina trihydrate,a fire-retardant smoke-suppressant filler. Triethyl phosphate has also been employed as a flame-resistant plasticizer in cellulose acetate.Because of its water solubility the use of triethyl phosphate is limited to situations where weathering resistance is unimportant.The halogenated alkyl phosphates are generally used for applications where lower volatility and greater resistance to leaching are required.공기와 물의 반응
Slowly dissolves in water with slight decomposition .반응 프로필
Organophosphates, such as Triethyl phosphate, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.건강위험
May be harmful by inhalation, ingestion or absorption. May cause irritation.화재위험
Special Hazards of Combustion Products: May produce hazardous decomposition products such as carbon dioxide, carbon monoxide and oxides of phosphorus.Safety Profile
Triethyl Phosphate is a flame retardant that exhibits low acute toxicity after oral, dermal or inhalation exposure. It is moderately toxic by ingestion, intraperitoneal, and intravenous routes. Experimental reproductive effects. Mutation data reported. Causes cholinesterase inhibition, but to a lesser extent than parathion. May be expected to cause nerve injury similar to that of other phosphate esters. Triethyl phosphate is stable under normal conditions of use. Avoid contact with strong bases and oxidizing agents. Triethyl phosphate is combustible at high temperatures. Heating to decomposition may release carbon dioxide, carbon monoxide, phosphorus oxides and other potentially toxic fumes or gases. Avoid heat, open flames and other potential sources of ignition.트리에틸인산염 준비 용품 및 원자재
원자재
준비 용품
BENZOFURAZAN-5-CARBONYL CHLORIDE
아세틸 케텐
METHYL 3-((PIPERIDIN-4-YL)METHYL)BENZOATE
Faropenem sodium hemipentahydrate
4-((1-(TERT-BUTOXYCARBONYL)PIPERIDIN-4-YL)METHYL)BENZOIC ACID
METHYL 4-((PIPERIDIN-4-YL)METHYL)BENZOATE
2,4-펜탄디온
2-((1-(TERT-BUTOXYCARBONYL)PIPERIDIN-4-YL)METHYL)BENZOIC ACID
무수초산
neutral size AKD
3-((1-(TERT-BUTOXYCARBONYL)PIPERIDIN-4-YL)METHYL)BENZOIC ACID
Cidofovir
아크릴산부탄
(4-CYANOBENZYL)PHOSPHONIC ACID DIETHYL ESTER
5-(BROMOMETHYL)-2,1,3-BENZOXADIAZOLE
Diethyl (trichloromethyl)phosphonate
METHYL 2-((PIPERIDIN-4-YL)METHYL)BENZOATE
(Phosphoric diethyl)N,N,N',N'-tetramethyldiamidophosphoric anhydride
메탄
케텐
ETHYL ACETATE, [1-14C]
트리에틸인산염 공급 업체
글로벌( 574)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17367 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 |
1026@dideu.com | China | 29322 | 58 |
| Zhangjiagang Xinya Chemical Co., Ltd. | +86-512-58980899 +8613915696858 |
sales@zjgxinyachem.com | China | 44 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418684 +8618949823763 |
sales@tnjchem.com | China | 25363 | 58 |
| Changyi Longchang Bio-Chemical Co., LTD | +86-13256193735 +86-13256193735 |
info@longchangchemical.com | China | 169 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7613 | 58 |
| Hangzhou Bayee Chemical Co., Ltd. | 0086-571-86990109 |
rachelhoo@bayeechem.com | China | 104 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |