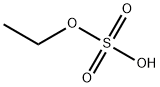
에틸 황산 C화학적 특성, 용도, 생산
화학적 성질
Colorless, oily liquid; boils at 280°C (536°F)(decomposes); density 1.367; highly solublein water; hydrolyzes forming sulfuric acid.
용도
Ethyl hydrogen sulfate is used as an intermediate in the synthesis of ethanol from ethylene.
일반 설명
ethyl hydrogen sulphate are colorless, oily liquids. They are soluble in water and weigh more than water. Contact with the material may cause severe irritation to skin, eyes, and mucous membranes. ethyl hydrogen sulphate may be toxic by ingestion, inhalation and skin absorption. ethyl hydrogen sulphate is used to make other chemicals.
공기와 물의 반응
They are soluble in water and weigh more than water. Heat may be generated by dissolution into water.
반응 프로필
Oxidizing acids are generally soluble in water with the release of hydrogen ions. The resulting solutions have pH's of less than 7.0. Materials in this group react with chemical bases (for example: amines and inorganic hydroxides) to form salts. These neutralization reactions occur as the base accepts hydrogen ions that the acid donates. Neutralizations can generate dangerously large amounts of heat in small spaces. The dissolution of acids in water or the dilution of their concentrated solutions with water may generate significant heat. The addition of water acids often generates sufficient heat in the small region of mixing to boil some of the water explosively. The resulting "bumping" spatters acid widely. These materials have significant ability as oxidizing agents. but that ability varies (for example, from high for nitric acid to low for sulfuric acid and most sulfonic acids). They can react with active metals, including iron and aluminum, and also many less active metals, to dissolve the metal and liberate hydrogen and/or toxic gases. Like other acids, materials in this group can initiate polymerization in certain classes of organic compounds. Their reactions with cyanide salts and compounds release gaseous hydrogen cyanide. Flammable and/or toxic gases are also often generated by their reactions with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and weak or strong reducing agents. Additional gas-generating reactions occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and even carbonates: the carbon dioxide gas from the last is nontoxic but the heat and spattering from the reaction can be troublesome. Acids often catalyze (increase the rate) of chemical reactions.
건강위험
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
화재위험
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
에틸 황산 준비 용품 및 원자재
원자재
준비 용품