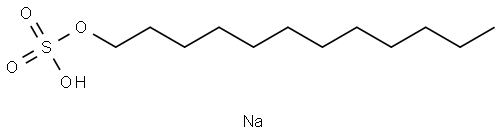
고급 알코올 황산에스테르염
|
|
고급 알코올 황산에스테르염 속성
- 녹는점
- 204-207 °C (lit.)
- 밀도
- 1.03 g/mL at 20 °C
- 인화점
- >100°C
- 저장 조건
- 2-8°C
- 용해도
- H2O: 0.1 M, 투명하거나 거의 투명함, 무색 내지 약간 노란색
- 물리적 상태
- 분말 또는 결정
- 색상
- 흰색에서 연한 노란색
- 수소이온지수(pH)
- 6-9 (10g/l, H2O, 20℃)
- 냄새
- 약간의 지방 냄새
- pH 범위
- 7.2
- 수용성
- ca. 150g/L(20℃)
- 최대 파장(λmax)
- λ: 260 nm Amax: 0.3
λ: 280 nm Amax: 0.2
- Merck
- 14,8636
- BRN
- 3599286
- InChIKey
- DBMJMQXJHONAFJ-UHFFFAOYSA-M
- LogP
- 1.600
- CAS 데이터베이스
- 151-21-3(CAS DataBase Reference)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,Xn,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38-36/38-22-11-21/22-42-41-20/21/22-37/38 | ||
| 안전지침서 | 26-36/37-36/37/39-36-22-39 | ||
| 유엔번호(UN No.) | UN 2926 4.1/PG 2 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | WT1050000 | ||
| F 고인화성물질 | 3 | ||
| TSCA | Yes | ||
| 위험 등급 | 4.1 | ||
| 포장분류 | III | ||
| HS 번호 | 29209010 | ||
| 유해 물질 데이터 | 151-21-3(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 1288 mg/kg (Walker) | ||
| 기존화학 물질 | KE-21884 |
고급 알코올 황산에스테르염 C화학적 특성, 용도, 생산
순도시험
(1) 알칼리도 : 이 품목 1.0g을 물 100mL에 녹여 페놀레드시액을 가한 후 0.1N 염산으로 적정할 때, 소비량은 0.5mL 이하이어야 한다.
(2) 염화나트륨 및 황산나트륨의 양 : 다음의 염화나트륨 및 황산나트륨의 시험법에 따라 시험할 때 염화나트륨 및 황산나트륨을 합한 양은 8.0% 이하이어야 한다.
① 염화나트륨 : 이 품목 약 5g을 정밀하게 달아 물 50mL에 녹이고 필요하면 묽은질산을 넣어 중성으로 한 다음 크롬산칼륨시액 2mL를 가하고 0.1N 질산은용액으로 적정한다. 따로 같은 방법으로 공시험을 한다.
0.1N 질산은용액 1mL = 5.844mg NaCl
② 황산나트륨 : 이 품목 약 1g을 정밀히 달아 물 10mL를 가해 가열하여 녹인 다음 뜨거울 때 에탄올 100mL를 가해 비점 부근에서 2시간 동안 가열한다. 뜨거울 때 유리여과기(G4)로 여과하고 침전물을 뜨거운 에탄올 100mL로 씻는다. 이어서 여과기내의 침전물에 물 약 150mL를 가하여 수세하여 녹이고 비이커에 받은 다음 염산 10mL를 넣어 끓을 때까지 가열한 후 염화바륨시액 25mL를 가하고 하룻밤 방치한다. 씻은 액이 염화물의 반응을 나타내지 아니할 때까지 씻은 다음 그 침전물을 건조하여 항량이 될 때까지 800℃에서 강열하여 황산바륨으로서 평량한다.
(3) 비황화알콜 : 이 품목 약 10 g을 정밀히 달아 물 100mL에 녹이고 에탄올 100mL를 가한 다음 분액깔때기로 옮기고 헥산 50mL로 3회 추출한다. 만일 현탁된 경우에는 염화나트륨을 가하여 층을 분리한다. 헥산을 모두 합하여 물 50mL씩으로 3회 씻고 무수황산나트륨으로 건조시킨 다음 수욕상에서 냄새가 나지 않을 때까지 헥산을 증발시키고 105℃에서 30분간 건조하고 평량할 때, 그 양은 4.0% 이하이어야 한다.
(4) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 5.0ppm 이하이어야 한다.
정 량 법 이 품목 약 5g을 정밀히 달아 물 150mL 및 염산 50mL를 넣고 환류냉각기를 달아 4시간 끓이고 식힌 다음 에테르 75mL씩으로 2회 추출하고 추출한 액을 합하여 수욕상에서 에테르를 날려 보낸 다음 105℃에서 30분간 건조하고 평량하여 이 잔류물을 총 알콜량으로 한다.
확인시험
(1) 이 품목의 수용액(1→10)은 확인시험법 중 나트륨염의 반응을 나타낸다.
(2) 이 품목의 수용액(1→10)을 염산으로 산성으로 하고 20분간 끊인 액은 확인시험법 중 황산염의 반응을 나타낸다.
정의
이 품목은 주로 라우릴황산나트륨[CH3(CH2)10CH2OSO3Na]으로 구성된 알킬황산나트륨의 혼합물이다.
개요
Sodium dodecyl sulfate is an anionic surfactant, and is a typical representative of sulphate-based surfactant. It is abbreviated as SDS, and also known as AS, K12, coco alcohol sulfate, sodium lauryl sulfate and foaming agent. The commercial products are usually white to light yellow crystalline powder. It is non-toxic, slightly soluble in alcohol, insoluble in chloroform and ether, soluble in water, and has good anionic and nonionic complex compatibility. It has good emulsibility, foamability, and foaming, infiltrating, decontaminating and dispersing properties. It is abundant in foams and quickly biodegradable, and has solubility next only to fatty alcohol polyoxyethylene ether sodium sulphate (abbreviated as AES). It is not sensitive to alkali and hard water, but its stability is inferior to general sulfonate under acidic conditions and is close to AES. It is not favorable to exceed 95 °C upon long-term heating, and its irritation is at the middle level among surfactants, with an irritation index of 3.3 for a 10% solution, which is higher than AES and lower than sodium dodecyl benzene sulfonate (abbreviated as LAS). Toxicity LD50 is 1300mg/kg. There is no evidence that this product is carcinogenic, but high doses may indeed irritate the skin. However, in general sanitary products the concentration is limited when used as a forming agent, and is in line with national standards. So there is no need to concern.Sodium dodecyl sulfate is a major component of detergent. It is usually used in the DNA extraction process to separate DNA after protein denaturation. It is often misread as sodium dodecyl sulfonate. It is widely used as a foaming agent in toothpaste, soap, shower gel, shampoo, detergent and cosmetics. 95% of personal care products and household cleaning products contain sodium lauryl sulfate.
The above information is edited by the chemicalbook of Jin Yinxue.
화학적 성질
Sodium lauryl sulfate consists of white or cream to pale yellow coloured crystals, flakes, or powder having a smooth feel, a soapy, bitter taste, and a faint odor of fatty substances. It is easily soluble in water.용도
Sodium dodecyl sulfate is an emulsifier and whipping aid that has a solubility of 1 g in 10 ml of water. It functions as an emulsifier in egg whites. It is used as a whipping aid in marshmallows and angel food cake mix. It also functions to aid in dissolving fumaric acid.생산 방법
Sodium lauryl sulfate is prepared by sulfation of lauryl alcohol, followed by neutralization with sodium carbonate.정의
ChEBI: Sodium dodecyl sulfate is an organic sodium salt that is the sodium salt of dodecyl hydrogen sulfate. It has a role as a detergent and a protein denaturant. It contains a dodecyl sulfate.일반 설명
White to pale yellow paste or liquid with a mild odor. Sinks and mixes with water.공기와 물의 반응
Insoluble in water.반응 프로필
DODECYL SULFATE is incompatible with strong oxidizers. Sodium dodecyl sulfate is also incompatible with cationic materials and with acids with pH below 2.5. Salts, basic, such as DODECYL SULFATE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.건강위험
Inhalation of dust causes sneezing and coughing. Ingestion of large amounts causes irritation of stomach. Dust irritates eyes and may cause burns on prolonged contact. Contact with skin causes some irritation; continued exposure to water solution causes drying out and cracking.화재위험
Flash point data for Sodium dodecyl sulfate are not available; however, Sodium dodecyl sulfate is probably combustible.Pharmaceutical Applications
Sodium lauryl sulfate is an anionic surfactant employed in a wide range of nonparenteral pharmaceutical formulations and cosmetics.It is a detergent and wetting agent effective in both alkaline and acidic conditions. In recent years it has found application in analytical electrophoretic techniques: SDS (sodium dodecyl sulfate) polyacrylamide gel electrophoresis is one of the more widely used techniques for the analysis of proteins; and sodium lauryl sulfate has been used to enhance the selectivity of micellar electrokinetic chromatography (MEKC).
색상 색인 번호
This anionic detergent is widely used in cosmetics and industry. As a skin irritant agent, SLS can be used in several dermatological applications. It is also a good indicator of excited skin during patch testing.Safety Profile
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion. An experimental teratogen. A human skin irritant. An experimental eye and severe skin irritant. A mild allergen. Mutation data reported. When heated to decomposition it emits toxic fumes of SO, and Na2O. See also ESTERS and SULFATES.Safety
Sodium lauryl sulfate is widely used in cosmetics and oral and topical pharmaceutical formulations. It is a moderately toxic material with acute toxic effects including irritation to the skin, eyes, mucous membranes, upper respiratory tract, and stomach. Repeated, prolonged exposure to dilute solutions may cause drying and cracking of the skin; contact dermatitis may develop.(3) Prolonged inhalation of sodium lauryl sulfate will damage the lungs. Pulmonary sensitization is possible, resulting in hyperactive airway dysfunction and pulmonary allergy. Animal studies have shown intravenous administration to cause marked toxic effects to the lung, kidney, and liver. Mutagenic testing in bacterial systems has proved negative.Adverse reactions to sodium lauryl sulfate in cosmetics and pharmaceutical formulations mainly concern reports of irritation to the skin or eyes following topical application.
Sodium lauryl sulfate should not be used in intravenous preparations for humans. The probable human lethal oral dose is 0.5–5.0 g/kg body-weight.
LD50 (mouse, IP): 0.25 g/kg
LD50 (mouse, IV): 0.12 g/kg
LD50 (rat, oral): 1.29 g/kg
LD50 (rat, IP): 0.21 g/kg
LD50 (rat, IV): 0.12 g/kg
저장
Sodium lauryl sulfate is stable under normal storage conditions. However, in solution, under extreme conditions, i.e. pH 2.5 or below, it undergoes hydrolysis to lauryl alcohol and sodium bisulfate.The bulk material should be stored in a well-closed container away from strong oxidizing agents in a cool, dry place.
Purification Methods
Purify this detergent by Soxhlet extraction with pet ether for 24hours, followed by dissolution in acetone/MeOH/H2O 90:5:5(v/v) and recrystallisation [Politi et al. J Phys Chem 89 2345 1985]. It has been purified by two recrystallisations from absolute EtOH, aqueous 95% EtOH, MeOH, isopropanol or a 1:1 mixture of EtOH/isopropanol to remove dodecanol, and dried under vacuum [Ramesh & Labes J Am Chem Soc 109 3228 1987]. SDS has also been purified by repeatedly foaming whereby a 0.15% aqueous solution is made to foam and the foam is discarded, then the H2O is removed in vacuo and the residue is diluted to the required concentrations [see Cockbain & McMullen Trans Faraday Soc 47 322 1951] or by liquid-liquid extraction [see Harrold J Colloid Sci 15 280 1960]. Dry it over silica gel. For DNA work it should be dissolved in excess MeOH passed through an activated charcoal column and evaporated until it crystallises out. It has also been purified by dissolving in hot 95% EtOH (14mL/g), filtering and cooling, then drying in a vacuum desiccator. Alternatively, it is crystallised from H2O, vacuum dried, washed with anhydrous Et2O and dried in vacuum again. These operations are repeated five times [Maritato J Phys Chem 89 1341 1985, Lennox and McClelland J Am Chem Soc 108 3771 1986, Dressik J Am Chem Soc 108 7567 1986]. [Beilstein 1 IV 1847.]비 호환성
Sodium lauryl sulfate reacts with cationic surfactants, causing loss of activity even in concentrations too low to cause precipitation. Unlike soaps, it is compatible with dilute acids and calcium and magnesium ions.Sodium lauryl sulfate is incompatible with salts of polyvalent metal ions, such as aluminum, lead, tin or zinc, and precipitates with potassium salts. Solutions of sodium lauryl sulfate (pH 9.5–10.0) are mildly corrosive to mild steel, copper, brass, bronze, and aluminum.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (dental preparations; oral capsules, suspensions, and tablets; topical and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.고급 알코올 황산에스테르염 준비 용품 및 원자재
원자재
준비 용품
고급 알코올 황산에스테르염 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| Hengshui Haoye Chemical Co.,Ltd. | +86-2102300 +86-18632882519 |
hy@chemcoms.com | China | 256 | 58 |
| Wuhan Boyuan Import & Export Co., LTD | +8615175982296 |
Mike@whby-chem.com | China | 974 | 58 |
| Hebei Yime New Material Technology Co., Ltd. | +86-66697723 +86-17703311139 |
admin@china-yime.com | China | 563 | 58 |
| Anhui Yiao New Material Technology Co., Ltd | +86-199-55145978 +8619955145978 |
sales8@anhuiyiao.com | China | 253 | 58 |
| Hebei Xinsheng New Material Technology Co., LTD. | +86-16632316109 |
xinshengkeji@xsmaterial.com | China | 1100 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7377 | 58 |
| Shandong Juchuang Chemical Co., LTD | +86-18885615001 +86-18885615001 |
admin@juchuangchem.com | China | 387 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-13474506593 +86-13474506593 |
sarah@tnjone.com | China | 794 | 58 |