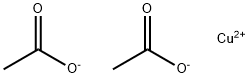
아세트산 제II구리, 무수
|
|
아세트산 제II구리, 무수 속성
- 녹는점
- 115°C
- 밀도
- 1.92 at 21.9℃
- 증기 밀도
- 6.9 (vs air)
- 증기압
- 0.002Pa at 25℃
- 저장 조건
- Inert atmosphere,Room Temperature
- 용해도
- DMSO (Slightly, Heated), Methanol (Slightly, Heated)
- 물리적 상태
- 가루
- 색상
- 녹색에서 파란색으로
- 수용성
- 물과 알코올에 용해됩니다. 에테르와 글리세롤에 약간 용해됩니다.
- 감도
- Hygroscopic
- Merck
- 14,2624
- BRN
- 3595638
- 노출 한도
- ACGIH: TWA 1 mg/m3
NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3
- 안정성
- 흡습성
- LogP
- -0.285 (est)
- 표면장력
- 72mN/m at 1.01-1.08g/L and 21.2-21.6℃
- 해리상수
- 4.69-4.89
- CAS 데이터베이스
- 142-71-2(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-36/37/38-50/53 | ||
| 안전지침서 | 26-60-61-37/39-29 | ||
| 유엔번호(UN No.) | UN 3077 9/PG 3 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | AG3480000 | ||
| F 고인화성물질 | 3-10 | ||
| TSCA | Yes | ||
| 위험 등급 | 9 | ||
| HS 번호 | 29152990 | ||
| 유해 물질 데이터 | 142-71-2(Hazardous Substances Data) | ||
| 독성 | mouse,LD50,intraperitoneal,2500ug/kg (2.5mg/kg),Biochemical Pharmacology. Vol. 30, Pg. 771, 1981. | ||
| 기존화학 물질 | KE-08897 |
아세트산 제II구리, 무수 C화학적 특성, 용도, 생산
개요
Copper (II) acetate, also referred to as cupric acetate, is the chemical compound with the formula Cu(OAc)22 where OAc- is acetate (CH3CO2-). The hydrated derivative, which contains one molecule of water for each Cu atom, is available commercially. Anhydrous Cu(OAc)2 is a dark green crystalline solid, whereas Cu2(OAc)4(H2O)2 is more bluish-green. Since ancient times, copper acetates of some form have been used as fungicides and green pigments. Today, copper acetates are used as reagents for the synthesis of various inorganic and organic compounds. Copper acetate, like all copper compounds, emits a blue-green glow in a flame.화학적 성질
Cupric acetate is a greenish Blue powder or small crystals.역사
Copper (II) acetate was historically prepared in vineyards, since acetic acid is a byproduct of fermentation. Copper sheets were alternately layered with fermented grape skins and dregs left over from wine production and exposed to air. This would leave a blue substance on the outside of the sheet. This was then scraped off and dissolved in water. The resulting solid was used as a pigment, or combined with arsenic trioxide to form copper acetoarsenite, a powerful insecticide and fungicide called Paris Green or Schweinfurt Green.During the Second World War copper acetate was used as shark repellent . Under war conditions, before adoption it has been tested only very briefly (while in general successfully). The source says copper acetate does repel sharks in some situations but not in all.
용도
Used as a catalyst or oxidizing agent in organic syntheses주요 응용
The uses for copper (II) acetate are more plentiful as a catalyst or oxidizing agent in organic syntheses. For example, Cu2(OAc)4 is used to couple two terminal alkynes to make a 1,3-diyne:Cu2(OAc)4 + 2 RC ≡ CH → 2 CuOAc + RC ≡ C-C ≡ CR + 2 HOAc
The reaction proceeds via the intermediacy of copper(I) acetylides, which are then oxidized by the copper(II) acetate, releasing the acetylide radical. A related reaction involving copper acetylides is the synthesis of ynamines, terminal alkynes with amine groups using Cu2(OAc)4.
일반 설명
A blue-green crystalline solid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Cupric acetate is used as an insecticide, in the preparation of other chemicals, as a fungicide, and mildew preventive.공기와 물의 반응
Water soluble.반응 프로필
Salts, basic, such as Cupric acetate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.건강위험
Inhalation of dust causes irritation of throat and lungs. Ingestion of large amounts causes violent vomiting and purging, intense pain, collapse, coma, convulsions, and paralysis. Contact with solutions irritates eyes; contact with solid causes severe eye surface injury and irritation of skin.화재위험
Special Hazards of Combustion Products: Irritating vapors of acetic acid may form in fires.Safety Profile
Poison by subcutaneous and intraperitoneal routes. Moderately toxic by ingestion. Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating fumes. See also COPPER COMPOUNDS.잠재적 노출
Cupric acetate is used as a fungicide, as a catalyst for organic reactions; in textile dyeing and as a pigment for ceramics.운송 방법
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Purification Methods
Recystallise it twice from warm dilute acetic acid solutions (5mL/g) by cooling. [Beilstein 2 IV 111.]비 호환성
Forms explosive materials with acetylene gas, ammonia, caustic solutions; sodium hypobromite; notromethane. Keep away from chemically active metals; strong acids; nitrates. Decomposes above 240C forming acetic acid fumes폐기물 처리
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill.아세트산 제II구리, 무수 준비 용품 및 원자재
원자재
준비 용품
N,N,N',N'-TETRAMETHYL-2-BUTENE-1,4-DIAMINE
Nabumetone
3-(Methylthio)butanal
Dye-fixing agent M
옥신-구리
플루페나믹 산
NETILMICIN 황산염
C.I. 디스퍼스 블루 3
4,6-Dimethylpyrimidine-5-carboxylic acid
2,6-DIMETHYL-3,5-HEPTANEDIONE
바닐린 아세테이트
meso-Tetrakis(4-chlorophenyl)porphyrin-Cu(II)
THIOACETANILIDE
2-methyl oxazole 4-ethyl ester
2-Iodo-4-nitroaniline
아세트산 제II구리, 무수 공급 업체
글로벌( 463)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Hunan aslsen technology co.,ltd | +8619310186019 |
aslsb@aslsen.com | China | 124 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 |
sales@alchempharmtech.com | United States | 63711 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 47465 | 58 |