MEPHOBARBITAL C화학적 특성, 용도, 생산
화학적 성질
White Crystalline Solid
용도
Controlled substance (depressant). Anticonvulsant; sedative; hypnotic
정의
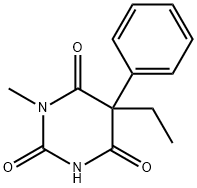
ChEBI: A member of the class of barbiturates, the structure of which is that of barbituric acid substituted at N-1 by a methyl group and at C-5 by ethyl and phenyl groups.
일반 설명
Mephobarbital, 3-methyl-5-ethyl-5-phenylbarbituric acid (metharbital), is metabolicallyN-demethylated to phenobarbital, which many consider toaccount for almost all of the activity. Its principal use is asan anticonvulsant.
Clinical Use
Mephobarbital is a barbiturate-derivative AED with a pKa of 7.7 (log P = 1.84 at pH 7.4). Approximately 50% of an oral dose of
mephobarbital is absorbed from the gastrointestinal tract. The plasma concentrations required for its therapeutic effects are
unknown. The principal route of mephobarbital metabolism is N-demethylation by the liver to form phenobarbital, which may be
excreted in the urine unchanged and as its p-hydroxy metabolite and glucuronide or sulfate conjugates.
Conversion to the 4-hydroxy metabolite is stereoselective, being catalyzed by either CYP2C19 (R-enantiomer) or CYP2B6
(S-enantiomer); individuals who are CYP2C19 poor metabolizers show decreased clearance. Approximately 75% of a
single oral dose of mephobarbital is converted to phenobarbital. It has not been determined whether mephobarbital contributes
to the antiseizure effect or whether it results from its active metabolite, phenobarbital. Similarly, it is unclear whether
mephobarbital, like phenobarbital, is a potent inducer of the enzymes involved in the metabolism of other drugs, but because
the drug is chemically and pharmacologically similar to phenobarbital and is metabolized to phenobarbital, this possibility is
likely.
Mephobarbital is less commonly used in the treatment of generalized and partial seizures. Like phenobarbital, it is classified as
a long-acting barbiturate. No evidence exists that it is more effective than phenobarbital in equivalent doses; however, it may
be less sedating in children.
Safety Profile
Poison by ingestion and
intraperitoneal routes. A human teratogen
by an unspecified route with developmental
abnormalities of the cardovascular
(circulatory) system. When heated to
decomposition it emits toxic NOx. See also
BARBITURATES.
MEPHOBARBITAL 준비 용품 및 원자재
원자재
준비 용품