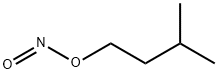
아질산 아이소아밀
|
|
아질산 아이소아밀 속성
- 끓는 점
- 99 °C(lit.)
- 밀도
- 0.872 g/mL at 25 °C(lit.)
- 증기압
- 35-59.995hPa at 20-25℃
- 굴절률
- n
20/D 1.386(lit.)
- 인화점
- 50 °F
- 저장 조건
- Store at +15°C to +25°C.
- 용해도
- 클로로포름, 메탄올(약간)에 용해됨
- 물리적 상태
- 액체
- 색상
- 맑은 노란색
- 수용성
- 18°C에서 <0.01g/100mL
- 감도
- Air & Light Sensitive
- Merck
- 14,5123
- BRN
- 969510
- 안정성
- 불안정한. 공기와 빛에 민감합니다. 가연성. 공기 또는 산소와 폭발성 혼합물을 형성합니다. 산화제, 환원제와 호환되지 않습니다.
- InChIKey
- OWFXIOWLTKNBAP-UHFFFAOYSA-N
- LogP
- 2.45-2.85
- CAS 데이터베이스
- 110-46-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-20/22 | ||
| 안전지침서 | 16-24-46 | ||
| 유엔번호(UN No.) | UN 1113 3/PG 2 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | NT0187500 | ||
| F 고인화성물질 | 1-8-9 | ||
| 자연 발화 온도 | 208 °C | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | II | ||
| HS 번호 | 29209090 | ||
| 유해 물질 데이터 | 110-46-3(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 505 mg/kg |
아질산 아이소아밀 C화학적 특성, 용도, 생산
물성
찻 주전자에 끓는 점: 99 °C (lit.)굴절 집게 스타일을: n20/D 1.386 (lit.)
깜박이는 점: 50 °F
스토리지 temp. : 2-8 °C
물 용해도: <0.01 그램/100 미리리터 at 18 °C
용도
의약품 중간체, 합성 물질 중간체.개요
Amyl nitrite had been used clinically as early as 1867, when the Scottish physician Sir Thomas Brunton used it as a vasodilator as treatment for angina pectoris in his patients. In the late 1880s, a protective effect on cyanide toxicity in canines was noted when amyl nitrite was inhaled postexposure. Amyl nitrite has been used clinically in a multicomponent cyanide antidote kit and is also a recreational drug of abuse (‘poppers’).화학적 성질
Isoamyl Nitrite is flammable and explosive and decomposes when exposed to air and sunlight.용도
Isoamyl nitrite is a light yellow, transparent liquid with a pleasant, fragrant, fruity odor. Amyl nitrite was introduced to medicine in 1859 and has been under considerable pharmacological investigation since that time. Its major use was for treating angina pectoris through its vasodilative effect on the coronary arteries. However, this effect is transient, and nitroglycerin and longer acting nitrates have largely replaced it. Amyl nitrite has been most helpful in clarifying the differential diagnosis of murmurs. For example, left ventricular outflowobstruction increases followingamyl nitrite administration. Mitral regurgitation decreases following amyl nitrite as does the apical diastolic rumble of mitral stenosis. The Austin–Flint rumble decreases followingamyl nitrite as does a ventricular septal defect and acyanotic tetralogy of Fallot. Pulmonic stenosis increases as does isolated valvular pulmonary stenosis following amyl nitrite.Isoamyl nitrite has also been reportedly used for inhalation abuse. The symptoms following inhalation of large doses by humans are flushing of the face, pulsatile headache, disturbing tachycardia, cyanosis (methemoglobinemia), weakness, confusion, restlessness, faintness, and collapse, particularly if the individual is standing. The symptoms are usually of short duration. Industrial intoxication has not been reported.
정의
ChEBI: Isoamyl nitrite is a nitrite ester having isopentyl as the alkyl group. It has a role as a vasodilator agent and an antihypertensive agent. It derives from an isoamylol.일반 설명
Clear yellow liquid.공기와 물의 반응
Highly flammable. Decomposes on exposure to air and light. Insoluble in water.반응 프로필
Isoamyl nitrite is an oxidizing agent but can serve as a reducing agent. Forms explosive mixtures with air or oxygen. .위험도
Flammable, dangerous fire risk, a strong oxidizer. Vapor may explode if ignited.화재위험
Isoamyl nitrite is flammable.잠재적 노출
Amyl nitrite is used to make pharmaceuticals; perfumes, diazonium compounds, and other chemicals.환경귀착
The primary mechanism of toxicity develops from the powerful oxidative effects of nitrites on hemoglobin. Methemoglobinemia, which develops when the iron atom in hemoglobin loses one electron to an oxidant, causing a change from the ferrous (2+) state to the ferric (3+) state, may cause cellular hypoxia. When methemoglobin levels exceed 10–15%, cyanosis may be evident. Nitrites also cause vasodilation by direct action on smooth muscle. Physical effects include decreases in blood pressure, headache, flushing of the face, increased heart rate, dizziness, and relaxation of involuntary muscles, especially of the blood vessels and the anal sphincter.Amyl nitrite may be irritating to the lungs and throat when breathed in. With exposure to the skin, amyl nitrite has irritant properties. It can also be readily absorbed, causing systemic effects with skin contact.
운송 방법
UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required.비 호환성
Vapors may form explosive mixture with air. Slowly decomposes in light, heat, and on contact with water. A strong oxidizer. Contact with reducing agents and easily oxidizable materials may cause fire and explosions. Reported to be an explosion hazard when exposed to air and light. Keep away from alcohols, antipyrine, alkaline materials; alkaline carbonates; potassium iodide; bromides, and ferrous salts. Attacks metals in the presence of moisture.폐기물 처리
Incineration with scrubber to remove nitrogen oxides from the combustion gases.아질산 아이소아밀 준비 용품 및 원자재
원자재
준비 용품
(5-Fluoropyridin-3-yl)methanol ,97%
2-Acetamido-5-fluoropyridine
METHYL 5-FLUOROPYRIDINE-3-CARBOXYLATE
2-Chloro-5-fluoropyridine
ETHYL 5-FLUORONICOTINATE
5-Fluoronicotinic acid
3-Amino-5-fluoropyridine
N,N-Diethyl-4-nitrosoaniline
니트로 BT
2-Amino-5-fluoropyridine
1,3-BENZODITHIOLYLIUM TETRAFLUOROBORATE
2-CHLORO-5-FLUOROISONICOTINIC ACID
2-CHLORO-2-HYDROXYIMINOACETIC ACID ETHYL ESTER
3-Fluoro-5-methylpyridine
2-broMo-5-cyclopropylpyridine
DIISOAMYLAMINE
N-NITROSO-N-ETHYLANILINE
ISOAMYL NITRATE
S-NITROSOGLUTATHIONE
아질산 아이소아밀 공급 업체
글로벌( 339)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Changzhou Zechong Biopharma Co., Ltd. | +undefined18621330623 |
sales@zechongchem.com | China | 28 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 |
sales@alchempharmtech.com | United States | 63711 | 58 |