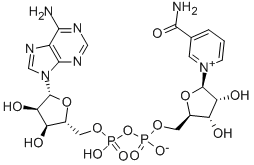
니코틴아미드아데닌 디뉴클레오티드
|
|
니코틴아미드아데닌 디뉴클레오티드 속성
- 녹는점
- 140-142 °C (decomp)
- 알파
- D20 -31.5° (c = 1.2 in water)
- 저장 조건
- -20°C
- 용해도
- H2O: 50 mg/mL
- 물리적 상태
- 가루
- 색상
- 하얀색
- 수소이온지수(pH)
- ~3.0 (50mg/mL in water)
- 냄새
- 냄새 없는
- 수용성
- 50mg/ml의 물에 용해됨
- Merck
- 14,6344
- BRN
- 3584133
- 안정성
- 안정적인. 흡습성. 강한 산화제와 호환되지 않습니다.
- InChIKey
- BAWFJGJZGIEFAR-WWRWIPRPSA-N
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,F,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36-68/20/21/22-20/21/22-40-22 | ||
| 안전지침서 | 36-26-36/37-24/25 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | UU3450000 | ||
| TSCA | Yes | ||
| HS 번호 | 29349990 | ||
| 기존화학 물질 | KE-25879 |
| 그림문자(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Warning | ||||||||||||||
| 유해·위험 문구: |
|
||||||||||||||
| 예방조치문구: |
|
니코틴아미드아데닌 디뉴클레오티드 C화학적 특성, 용도, 생산
존재
생물체에서 NAD는 아미노산인 트립토판이나 아스파르트산으로부터 합성될 수 있다. 다른 방식으로 조효소의 보다 복잡한 구성 성분들이 니아신처럼 음식물로부터 섭취된다. 유사한 화합물들이 NAD의 구조를 파괴하는 반응에 의해 방출된다. 그런 다음 이들 예비 화합물들은 회수 경로를 거치면서 활성형으로 재활용된다. 일부 NAD는 니코틴아마이드 아데닌 다이뉴클레오타이드 인산(NADP)으로 전환된다.개요
니코틴아마이드 아데닌 다이뉴클레오타이드는 모든 살아있는 세포에서 발견되는 보조 인자이다. 니코틴아마이드 아데닌 다이뉴클레오타이드는 인산기를 통해 결합된 두 개의 뉴클레오타이드로 구성되어 있기 때문에 다이뉴클레오타이드라고 불린다. 뉴클레오타이드 중 하나는 아데닌 핵염기를 가지고, 다른 하나는 니코틴아마이드를 가지고 있다. 니코틴아마이드 아데닌 다이뉴클레오타이드는 산화형과 환원형의 두 가지 형태로 존재하며, 산화형은 NAD+, 환원형은 NADH로 약칭된다.용도
물질대사에서 니코틴아마이드 아데닌 다이뉴클레오타이드는 산화환원 반응에 관여하며, 한 반응에서 다른 반응으로 전자를 전달한다. 따라서 니코틴아마이드 아데닌 다이뉴클레오타이드는 세포에서 두 가지 형태로 발견된다. NAD+는 산화제로서 다른 분자로부터 전자를 받아서 환원된다. 환원된 NAD+는 NADH가 되어 전자를 공여할 수 있는 환원제로 사용될 수 있다. 이러한 전자전달 반응은 니코틴아마이드 아데닌 다이뉴클레오타이드의 주요 기능이다. 또한 이것은 다른 세포 과정에서도 사용되며, 특히 번역 후 변형에서 단백질로부터 작용기를 추가하거나 제거하는 효소들의 기질에 사용된다. 이러한 기능의 중요성 때문에 NAD 대사에 관여하는 효소들은 신약 개발의 표적이 된다.개요
β-Nicotinamide adenine dinucleotide (NAD+) plays a major role in metabolism as a cofactor and mobile electron acceptor. NAD+ is a required oxidizing cosubstrate for many enzymes. It is reduced to NADH (Cat# N-035) which carries electrons to the electron transport chain for subsequent oxidative phorphorylation and ATP production. NAD+ is capable of donating ADP-ribose moieties to a protein, producing nicotinamide in the process. Sirtuin enzymes use NAD+ as a substrate to deacetylate proteins and direct activity between the nucleus and mitochondria. NAD+ is regenerated by fermentation and by oxidative phosphorylation.화학적 성질
Beta-Nicotinamide adenine dinucleotide is a hygroscopic white powder. It should be stored desiccated. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Recommended storage temperature -20°C.역사
The coenzyme NAD+ was first discovered by British biochemists Arthur Harden and William John Young in 1906. They observed that the addition of boiled and filtered yeast extract significantly increased the rate of alcoholic fermentation in unboiled yeast extracts. They named the unidentified factor responsible for this effect a coferment. By extensively purifying it from yeast extracts, Hans von Euler-Chelpin identified this heat-stable factor as a nucleotide sugar phosphate. In 1936, German scientist Otto Heinrich Warburg demonstrated the role of this nucleotide coenzyme in hydride transfer and determined the nicotinamide portion as the site of redox reactions. Vitamin precursors of NAD+ were first identified in 1938, when Conrad Elvehjem showed that liver has an "anti-black tongue" activity in the form of nicotinamide. Then, in 1939, he provided the first strong evidence that niacin is used to synthesize NAD+.정의
ChEBI: β-Nicotinamide adenine dinucleotide (NAD) is the oxidized form of β-Nicotinamide Adenine Dinucleotide. It exists as an anion under normal physio-logic conditions. It is functionally related to a deamido-NAD zwitterion. It is a conjugate base of a NAD(+). It is found widely in nature and is involved in numerous enzymatic reactions in which it serves as an electron carrier by being alternately oxidized (NAD+) and reduced (NADH). (Dorland, 27th ed)주요 응용
β-Nicotinamide adenine dinucleotide (β-NAD) is a cofactor of alcohol dehydrogenase and acts as a neuromodulator and an inhibitory neurotransmitter in visceral smooth muscles. The NAD/NADH ratio has a role in the regulation of intracellular redox potential. It thereby influences metabolic reactions in vivo. It has been used for the preparation of deacetylated tubulin. It has also been used for UDP-glucose-6-hydrogenase (UGDH) enzyme activity assay of orital fibroblast cell lysates.β-Nicotinamide adenine dinucleotide (NAD+) and β-Nicotinamide adenine dinucleotide, reduced (NADH) comprise a coenzyme redox pair (NAD+:NADH) involved in a wide range of enzyme catalyzed oxidation reduction reactions. In addition to its redox function, NAD+/NADH is a donor of ADP-ribose units in ADP-ribosylaton (ADP-ribosyltransferases; poly(ADP-ribose) polymerases ) reactions and a precursor of cyclic ADP-ribose (ADP-ribosyl cyclases).
일반 설명
β-Nicotinamide adenine dinucleotide (NAD) is a ubiquitously found electron carrier and a cofactor. NAD+ contains an adenylic acid and a nicotinamide-5′-ribonucleotide group linked together by a pyrophosphate moiety. In NAD+ complexes, the enzyme-cofactor interactions are highly conserved.생물학적 활성
NAD+, known more formally as nicotinamide adenine dinucleotide, is a signaling molecule as well as a cofactor or substrate for many enzymes. It acts as an oxidizing agent, accepting electrons from other molecules while being converted to its reduced form, NADH. NAD+ is also essential for the activity of several enzymes, including poly(ADP)-ribose polymerases and cADP-ribose synthases. For example, it is used by some sirtuins to mediate protein deacetylation, producing O-acetyl-ADP-ribose and nicotinamide as well as the deacetylated protein.부작용
In the studies conducted thus far, people taking between 1000mg-2000mg per day have reported zero long term side effects. However, during the active infusion, some people may feel temporary nausea or stomach discomfort.Purification Methods
NAD is purified by paper chromatography or better on a Dowex-1 ion-exchange resin. The column is prepared by washing with 3M HCl until free of material absorbing at 260nm, then with water, 2M sodium formate until free of chloride ions and, finally, with water. NAD, as a 0.2% solution in water, is adjusted with NaOH to pH 8, and adsorbed onto the column, washed with water, and eluted with 0.1M formic acid. Fractions with strong absorption at 360nm are combined, acidified to pH 2.0 with 2M HCl, and cold acetone (ca 5L/g of NAD) is added slowly and with constant agitation. It is left overnight in the cold, then the precipitate is collected in a centrifuge, washed with pure acetone and dried under vacuum over CaCl2 and paraffin wax shavings [Kornberg Methods Enzymol 3 876 1957]. It has been purified by anion-exchange chromatography [Dalziel & Dickinson Biochemical Preparations 11 84 1966.] The purity is checked by reduction to NADH (with EtOH and yeast alcohol dehydrogenase) which has 340mn 6220 M-1cm-1. [Todd et al. J Chem Soc 3727, 3733 1957.] [pKa, Lamborg et al. J Biol Chem 231 685 1958.] The free acid crystallises from aqueous Me2CO with 3H2O and has m 140-142o. It is stable in cold neutral aqueous solutions in a desiccator (CaCl2) at 25o, but decomposes at strong acid and alkaline pH. Its purity is checked by reduction with yeast alcohol dehydrogenase and EtOH to NADH and noting the OD at 340nm. Pure NADH (see below) has 340 6.2 x 104 M-1cm-1, i.e. 0.1mole of NADH in 3mL and in a 1cm path length cell has an OD at 340nm of 0.207. [Beilstein 26 IV 3644.]참고 문헌
1. Pollak N.; D?lle C.; Ziegler M. The power to reduce: pyridine nucleotides - small molecules with a multitude of functions. Biochem. J. 2007, 402(2), 205-18. DOI:10.1042/BJ200616382. Unden G, Bongaerts J. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim. Biophys. Acta. 1997, 1320(3), 217-34. DOI:10.1016/S0005-2728(97)00034-0
3. Houtkooper, R.H., Cantó, C., Wanders, R.J., et al. The secret life of NAD+: An old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 31(2), 194-223 (2010). DOI:10.1210/er.2009-0026
4. Experimental and theoretical electron density studies in large molecules: NAD+, beta-nicotinamide adenine dinucleotide DOI:10.1021/JP034478B
5. A Mechanism of Adsorption of beta-Nicotinamide Adenine Dinucleotide on Graphene Sheets: Experiment and Theory DOI:10.1002/chem.200900399
6. β-Nicotinamide Adenine Dinucleotide (β-NAD) Inhibits ATP-Dependent IL-1β Release from Human Monocytic Cells DOI:10.3390/ijms19041126
7. Oxidation of β-Nicotinamide Adenine Dinucleotide (NADH) by Au Cluster and Nanoparticle Catalysts Aiming for Coenzyme Regeneration in Enzymatic Glucose Oxidation DOI:10.1021/acssuschemeng.0c01893
니코틴아미드아데닌 디뉴클레오티드 준비 용품 및 원자재
원자재
Concentrated hydrochloric acid
Resin column
Anion exchange resin,strong basic quarternary ammoniumⅠ
AMBERLITE(R) IRC-50
Resin 201
Brown
Ion exchange resin column
Spectrophotometer
Cation exchange resin,weak acidic acrylic acid
Cation exchange resin,strong acidic styrene 001×7×7
염화칼륨
효모 추출물
암모니아(가스)
준비 용품
니코틴아미드아데닌 디뉴클레오티드 공급 업체
글로벌( 761)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Maintain Biotech Co., Ltd. | +86-75589664337 +86-15112661142 |
cami@maintain-bio.com | China | 66 | 58 |
| SyncoZymes (Shanghai) Co., Ltd., | +86-021-68187180-811 +86-13681683526 |
lchen@syncozymes.com | China | 188 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 |
xie@china-sinoway.com | China | 992 | 58 |
| Xiamen Wonderful Bio Technology Co., Ltd. | +8613043004613 |
Sara@xmwonderfulbio.com | China | 305 | 58 |
| Henan Tengmao Chemical Technology Co. LTD | +8615238638457 |
salesvip2@hntmhg.com | China | 415 | 58 |
| Mainchem Co., Ltd. | -- |
sarah@mainchem.com | China | 6572 | 58 |
| Hebei Yalin Technology Co., Ltd | +86-19322905052 +86-19322905052 |
yl01@ylmetallurgy.com | China | 9 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 |
admin@guyunchem.com | China | 616 | 58 |
| Shanghai Affida new material science and technology center | +undefined15081010295 |
2691956269@qq.com | China | 359 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
니코틴아미드아데닌 디뉴클레오티드 관련 검색:
아세틸 보조 효소 A 조효소 A 니코틴산아미드 아데닌 6-티오구아닌 유비데카레논 퓨린 니코틴아미드아데닌 디뉴클레오티드
NADP sodium salt
Triphosphopyridine nucleotide
2,3-Dimethoxy-5-methyl-p-benzoquinone
NADP, Disodium Salt
Adenine phosphate salt(1:1)
NADH, disodium salt
Nicotinamide hypoxanthine dinucleotide phosphate reduced tetrasodium salt
β-Nicotinamide Mononucleotide
β-nicotinamide mononucleotide, reduced form, disodium salt(NMNH)
2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride