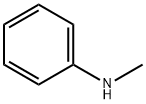
N-메틸아닐린
|
|
N-메틸아닐린 속성
- 녹는점
- -57 °C (lit.)
- 끓는 점
- 196 °C (lit.)
- 밀도
- 0.989 g/mL at 25 °C (lit.)
- 증기압
- 0.5 hPa (20 °C)
- 굴절률
- n
20/D 1.571(lit.)
- 인화점
- 174 °F
- 저장 조건
- Store below +30°C.
- 용해도
- 물: 경미하게 녹는30g/L
- 산도 계수 (pKa)
- 4.84(at 25℃)
- 물리적 상태
- 액체
- 색상
- 투명한 노란색에서 갈색까지
- 수소이온지수(pH)
- 7.6 (1g/l, H2O, 20℃)
- 냄새
- 아닐린 같은 냄새
- 수용성
- 30g/L
- 감도
- Air Sensitive
- Merck
- 14,6019
- BRN
- 741982
- Henry's Law Constant
- (x 10-5 atm?m3/mol): 1.19 at 25 °C (approximate - calculated from water solubility and vapor pressure)
- 노출 한도
- NIOSH REL: TWA 0.5 ppm (2 mg/m3), IDLH 100 ppm; OSHA PEL: TWA 2 ppm (9 mg/m3); ACGIH TLV: TWA 0.5 ppm (adopted).
- Dielectric constant
- 6.0(22℃)
- 안정성
- 강한 산화제와 호환되지 않습니다. 공기에 노출되면 변색됩니다.
- InChIKey
- AFBPFSWMIHJQDM-UHFFFAOYSA-N
- LogP
- 1.66 at 20℃
- CAS 데이터베이스
- 100-61-8(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 23/24/25-33-50/53 | ||
| 안전지침서 | 28-36/37-45-60-61-28A | ||
| 유엔번호(UN No.) | UN 2294 6.1/PG 3 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | BY4550000 | ||
| F 고인화성물질 | 8 | ||
| 자연 발화 온도 | 500 °C | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | III | ||
| HS 번호 | 29214200 | ||
| 유해 물질 데이터 | 100-61-8(Hazardous Substances Data) | ||
| 독성 | LD in rabbits (g/kg): 0.28 orally; in rabbits, cats (mg/kg): 24, 24 i.v. (Treon) | ||
| IDLA | 100 ppm | ||
| 기존화학 물질 | KE-23449 | ||
| 유해화학물질 필터링 | 97-1-183 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 알킬 아닐린과 그 염류 및 이를 25% 이상 함유한 혼합물 |
N-메틸아닐린 C화학적 특성, 용도, 생산
화학적 성질
N-Methylaniline is a yellow to light brown oily liquid with a weak, ammonia-like odor. soluble in ethanol, ether, chloroform, slightly soluble in water. Turns reddishbrown if left standing.물리적 성질
Colorless to yellow to pale brown liquid with a faint, ammonia-like odor. Odor threshold concentration is 1.7 ppm (quoted, Amoore and Hautala, 1983).용도
N-Methylaniline is used as a solvent and in organic synthesis. It is used in the production of cationic brilliant red FG, cationic pink B, reactive yellow brown KGR, etc. in the dye industry. N-methylaniline is an intermediate in the synthesis of the insecticide buprofezin and the herbicide mefenacet.용도
N-methylaniline was used as raw material, and metal-organic skeleton material CAU-10pydc was used as catalyst to catalyze the reaction of CO2 with n-methylaniline to produce N-methyl-N-phenylformamide in mild conditions and green solvent acetonitrile[1]. Poly (N-methylaniline) (PNMA) was prepared by the electrooxidation of N-methylaniline in acidic aqueous solution. PNMA prepared in acidic aqueous solution with different organic solvents and anions had different conductivity. Such as the experimental group added acetonitrile, N, N-dimethylformamide and dimethylsulfoxide, and the experimental group which adding dimethylsulfoxide had the strongest conductivity[2].References:
[1] Zhiqiang Wang, Chenghua Deng, Xiao Liu, Wenmin Wang, Highly efficient conversion of CO2 into N-formamides catalyzed by a noble-metal-free aluminum-based MOF under mild conditions, 2023, 52, 11163-11167.
[2] Jun YANO , Hiroko YOSHIKAWA , Tomomi MUKAI , Sumio YAMASAKI , Akira KITANI, Effect of Anions and Added Organic Solvents of Polymerizing Solutions on the Conductivity of Poly (N-methylaniline), 2006, 74, 42-48.
제조 방법
N-methylaniline was synthesized by the reaction of aniline with dimethyl sulfate. Dimethyl sulfate was added dropwise to the mixed solution of aniline and water below 10°C, stirred for 1 h, and then added dropwise with 30% sodium hydroxide solution. The upper layer is the organic phase, and the lower layer is extracted with benzene. After the benzene is recovered from the extract, the obtained oil Chemicalbook-like substance is combined with the organic phase to obtain a mixture of aniline, N-methylaniline and N,N-dimethylaniline. The mixture was treated with sulfuric acid, and the aniline formed sulfate crystals which were filtered off. N,N-dimethylaniline can be converted to N-methylaniline by the following reaction.정의
ChEBI: N-methylaniline is a methylaniline that is aniline carrying a methyl substituent at the nitrogen atom. It is a phenylalkylamine, a secondary amine and a methylaniline. It derives from an aniline.일반 설명
Chemical oxidation of N-methylaniline with dichromate (oxidant) has been studied by Raman spectroscopy.위험도
Toxic by ingestion, inhalation, and skin absorption. Methemoglobinemia and central nervous system impairment.건강위험
Recommended Personal Protective Equipment: Approved respirator; rubber gloves; splash proof goggles; Symptoms Following Exposure: Inhalation causes dizziness and headache. Ingestion causes bluish discoloration (cyanosis) of lips, ear lobes, and fingernail beds. Liquid irritates eyes. Absorption through skin produces same symptoms as for ingestion; General Treatment for Exposure: INHALATION: remove victim to fresh air and call a physician at once; administer oxygen until physician arrives. INGESTION: give large amount of water; get medical attention at once. EYES or SKIN: flush with plenty of water for at least 15 min.; if cyanosis is present, shower with soap and warm water, with special attention to scalp and finger nails; remove any contaminated clothing; Toxicity by Inhalation (Threshold Limit Value): Data not available; Short-Term Inhalation Limits: Data not available; Toxicity by Ingestion: Data not available; Late Toxicity: Data not available; Vapor (Gas) Irritant Characteristics: Data not available; Liquid or Solid Irritant Characteristics: Data not available; Odor Threshold: Data not available.화학 반응
Reactivity with Water No reaction; Reactivity with Common Materials: May attack some forms of plastic; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Safety Profile
Poison by ingestion and intravenous routes. Moderately toxic by subcutaneous route. When heated to decomposition it emits toxic fumes of NOx.잠재적 노출
The material is used as an intermediate in organic synthesis, as a solvent and as an acid acceptorCarcinogenicity
N-methyl aniline (1.95 g/kg of food) given together with sodium nitrite (1.0 g/l of drinking water) to Swiss mice resulted in a 17% incidence of lung adenomas and a 14% incidence of malignant lymphomas; there were no carcinogenic effects in animals treated with Nmethyl aniline alone, suggesting that in vivo nitrosation is necessary for forming carcinogenic nitrosamines.In bacterial mutagenicity assays N-methyl aniline was negative with or without metabolic activation.
환경귀착
Soil. Reacts slowly with humic acids or humates forming quinoidal structures (Parris, 1980).운송 방법
UN2294 N-Methylaniline, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.Purification Methods
Dry it with KOH pellets and fractionally distil it under vacuum. Acetylate, and the acetyl derivative is recrystallised to constant melting point (m 101-102o), then hydrolysed with aqueous HCl and distilled from zinc dust under reduced pressure. [Hammond & Parks J Am Chem Soc 77 340 1955, Beilstein 12 IV 241.]폐기물 처리
Controlled incineration whereby oxides of nitrogen are removed from the effluent gas by scrubber, catalytic or thermal device.N-메틸아닐린 준비 용품 및 원자재
원자재
준비 용품
ASTRAZON PINK FG
2-Chloro-N-(2,6-dimethylphenyl)acetamide
말라카이트그린(옥살산염)
4-(TRIMETHYL-UREIDO)-BENZENESULFONYL CHLORIDE
N-(2-Hydroxyethyl)-N-methylaniline
N-METHYL-N-PHENYLCARBAMOYL CHLORIDE
RIBITYL-3,4-XYLIDINE*
청색 054 기초염료
REACTIVE BLUE 4
MICHLER'S 케톤
벤조티아졸
1H-PYRROLO[2,3-B]PYRIDINE, 2-METHYL-
크리스탈 바이올렛
메틸 오렌지
헥사메틸다이사이록산
N-METHYL-N-BENZYLANILINE
4-(DIMETHYLAMINO)PHENYL THIOCYANATE
메틸렌블루
N-Chloromethyl-N-phenylaminoformyl chloride
브푸로페진
P-다이메틸아미노벤즈알데하이드
ASTRAZON BRILLIANT RED 4G
N-METHYL-N-PHENYL-2-PIPERAZIN-1-YLACETAMIDE
N-2,4-DIMETHYLPHENYL-N'-METHYLFORMAMIDINE
5-Azaindole
아세토초산 o-아니시다이드
플루시토신
Acid Yellow 127
2-아미노-4,6-디메톡시피리미딘
바닐린
2,6-다이메틸아닐린
4-(Dimethylamino)benzophenone
메틸(1-)-1-페닐히드라진
리도카인
메페나셋
1,2-DIPHENYLVINYLENE CARBONATE
N-메틸아닐린 공급 업체
글로벌( 431)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17367 | 58 |
| Tianjin Zhongxin Chemtech Co., Ltd. | +86-022-66880623 +8618622897568 |
sales@tjzxchem.com | China | 559 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 |
daisy@crovellbio.com | China | 5964 | 58 |
| Yancheng Green Chemicals Co.,Ltd | +undefined-86-25-86655873 |
info@royal-chem.com | China | 114 | 58 |
| Changde Changlian Chemical Co., Ltd. | +86-25-86736270 +86-15905172087 |
brian.he@royal-chem.com | China | 103 | 58 |
| Wuhan Boyuan Import & Export Co., LTD | +8615175982296 |
Mike@whby-chem.com | China | 974 | 58 |
| Gansu Jinyuantai New Material Co., Ltd. | +86-025-86618065 +86-13914714331 |
sales@jinyuantaichem.com | China | 73 | 58 |
| Ningxia Jinhua Chemical Co.,Ltd | 025-52279164 |
info@nxjhchem.com | China | 79 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12453 | 58 |