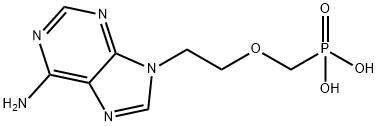
Adefovir
|
|
Adefovir 속성
- 녹는점
- >260°C
- 끓는 점
- 632.5±65.0 °C(Predicted)
- 밀도
- 1.88
- 저장 조건
- -20°C
- 용해도
- 0.1M NaOH: ≥5mg/mL
- 산도 계수 (pKa)
- pKa1 2.0, pKa2 6.8(at 25℃)
- 물리적 상태
- 가루
- 색상
- 흰색에서 베이지색
- InChIKey
- SUPKOOSCJHTBAH-UHFFFAOYSA-N
- CAS 데이터베이스
- 106941-25-7(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 25 | ||
| 안전지침서 | 45 | ||
| 유엔번호(UN No.) | 2811 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | SZ6563500 | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | Ⅲ | ||
| HS 번호 | 29339900 | ||
| 유해 물질 데이터 | 106941-25-7(Hazardous Substances Data) |
Adefovir C화학적 특성, 용도, 생산
화학적 성질
Pale Brown Solid용도
Adefovir has been used to study its anti-retro viral effect on porcine endogenous retrovirus (PERV) activity.원료
It has a lower propensity to induce drug resistance than lamivudine. Clinical trials of patients receiving 48 weeks of therapy did not identify any cases of resistance. Longer courses yield resistant strains of HBV with mutations in the DNA polymerase gene; other rare variants of resistant strains have been identified. Lamivudine-resistant strains of HBV retain susceptibility to adefovir.Pharmaceutical Applications
A nucleotide analog of adenosine monophosphate, administered orally as its prodrug, adefovir dipivoxil.Pharmacokinetics
Oral absorption: c. 60%Cmax 10 mg/kg oral: 18.4 ng/mL
Plasma half-life: c. 7.5 h.
Volume of distribution: 392 mL/kg
Plasma protein binding: Not known
The prodrug is metabolized to adefovir, which is excreted by the kidneys and therefore requires dose adjustment in patients with impaired renal function. It does not induce cytochrome P450 at standard doses and does not influence the metabolism or plasma concentrations of the other licensed medications used in the treatment of hepatitis B.
Clinical Use
Treatment of chronic hepatitis B virus infection in patients >12 years of age부작용
It is generally well tolerated, with headache, pharyngitis, abdominal pain and peripheral neuropathy being the most common side effects. Nephrotoxicity has been observed in some patients, with those receiving higher doses and longer courses of therapy at greater risk. Exacerbation of hepatitis has been reported in patients immediately following discontinuation of treatment. Most exacerbations occur within 12 weeks of stopping therapy, and elevations of alanine aminotransferase (ALT) up to 10 times the upper limit of normal can be observed in over 25% of patients. Lactic acidosis has been reported in a few patients and is an indication for immediate discontinuation.Adefovir 준비 용품 및 원자재
원자재
준비 용품
Adefovir 공급 업체
글로벌( 402)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 |
sales02@airuikechemical.com | China | 994 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 |
admin@nexconn.com | China | 7836 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5909 | 58 |
Adefovir 관련 검색:
6-아미노 카프로산 P-아니시딘 트리스(하이드록시메틸)아미노메탄 메톡시아세트 산 트리플르오르메톡시벤젠 아데닌 아니졸 글리신 퓨린 글리콜 카보네이트
Methoxydiethylborane
m-Anisyl alcohol
ALTRENOGEST
Gemcitabine hydrochloride
Adefovir Dipivoxil Impurity 20
[[2-(6-Amino-9H-purin-9-yl)ethoxy]methyl]phosphonic acid diethyl ester
Adefovir iMpurity
Adefovir Dipivoxil Impurity 4