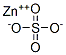황산아연 C화학적 특성, 용도, 생산
물성
물에 녹기 쉽고 소독성, 수렴성이 있어서 의약, 방부제, 매염제로 쓰거나 아연 비료로 이용됨.
화학 반응
물에 쉽게 녹으며 수렴성이 있어서
의약, 방부제, 매염제 로 쓰이거나
아연 비료로 쓰이기도 한다.
용도
1) 안료(리토폰 및 습식 아연화, 활성 아연백 제조용).
2) 사라사 날염용 매염제.
3) 인견, 스프 및 셀로판 응고 중탕용.
4) 목재 등의 방부용.
5) 6국 기재의 의약품 : 수렴성, 방부성, 지혈성을 이용하여 세정제, 함수제, 주입제 또 안과약으로서 외용한다.
화학적 성질
used in zinc plating and as a mordant [KIR84]
물리적 성질
The anhydrous sulfate is a colorless rhombohedral crystalline solid; refractive index 1.658; density 3.54 g/cm
3; decomposes at 600°C; soluble in water, methanol, and glycerol.
The heptahydrate, ZnSO
4?7H
2O, is a colorless crystalline solid; metallic taste; rhombohedral crystals; effloresces; refractive index 1.457; density 1.957 g/cm
3 at 25°C; melts at 100°C; loses all its water molecules at 280°C; decomposes above 500°C; very soluble in water, 96.5 g/100mL at 20°C; soluble in glycerol, 40 g/100 mL; insoluble in alcohol
The hexahydrate, ZnSO
4?6H
2O constitutes colorless monoclinic or tetragonal crystals; density 2.072 g/cm
3at 15°C; loses five water molecules at 70°C; soluble in water.
용도
The principal
commercial preparation of zinc sulfate is the monohydrate
granular (36% of zinc) used as a fertilizer. It is also a
component of spinning bath in the production of rayon,
carbamate fungicides, zinc plating baths, and ophthalmic
solutions. Zinc sulfate is used as an accelerating agent in
dental impression material, froth flotation agent, and animal
feed additive.
정의
zinc sulphate: A white crystalline water-soluble compound made by heating zinc sulphide ore in air and dissolving out and recrystallizing the sulphate. The common form is the heptahydrate, ZnSO
4.7H
2O; r.d. 1.9. This loses water above 30°C to give the hexahydrate and more water is lost above 70°C to form the monohydrate. The anhydrous salt forms at 280°C and this decomposes above 500°C. The compound, which was formerly called white vitriol, is used as a mordant and as a styptic (to check bleeding).
생산 방법
Zinc sulfate is produced from side streams of electrolytic
zinc manufacture. The main source of secondary zinc for
zinc sulfate production is galvanizer residues. ZnSO4 is
available as the mono-, hexa- and heptahydrates with zinc
contents of 36%, 24%, and 22%, respectively.
일반 설명
Anhydrous Zinc sulphate is a colorless crystalline solid. Zinc sulphate is also obtained as a hexahydrate, ZnSO4.6H2O, and as a heptahydrate ZnSO4.7H2O. All forms are soluble in water. All are noncombustible. The primary hazard is the threat posed to the environment. Immediate steps should be taken to limit its spread to the environment. Zinc sulphate is used in the production of rayon, as a feed supplement, used to obtaine lectrolyte zinc, in printing textiles and to make lithopone, to impregnate wood and hides,as an additive to spinning baths for production of synthetic silks, in electroplating, and in animal feeds.
공기와 물의 반응
Water soluble. Efflorescent in air. Aqueous solutions are acidic.
반응 프로필
Acidic salts, such as Zinc sulphate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
건강위험
Inhalation of dust causes irritation of nose and throat. Ingestion can cause irritation or corrosion of the alimentary tract. Contact with eyes or skin causes irritation.
농업용
White vitriol is another name for zinc sulphate
heptahydrate, and is a commonly used zinc salt. It is
widely used as a fertilizer for overcoming zinc
deficiency.
공업 용도
Ferro sulfate (FeSO
4·7H
2O) is a crystalline substance greenish in color, with a specific
gravity of 1.899. Ferro sulfate is obtained from various solutions using a vacuum crystallization
method. Ferro sulfate has been used as a depressant and co-depressant in
the following applications: (a) depression of sphalerite together with cyanide ,
(b) depression of fine molybdenite also with cyanide, and (c) in copper/lead separation
using a method, based on copper depression by cyanide.
Safety Profile
Poison by ingestion,
intraperitoneal, subcutaneous, and
intravenous routes. Human systemic effects
by ingestion: acute pulmonary edema,
agranulocytosis, blood pressure decrease,
diarrhea and other gastrointestinal changes,
hypermotility, increased pulse rate without
blood pressure decrease, level changes for
metals other than Na/K/Fe/Ca/P/Cl,
microcytosis with or without anemia,
normocytic anemia. Experimental
teratogenic and reproductive effects.
Questionable carcinogen with experimental
tumorigenic data. Human mutation data
reported. An eye irritant. When heated to
decomposition it emits toxic fumes of SOx
and ZnO. See also SULFATES and ZINC
COMPOUNDS.
Purification Methods
Crystallise it from aqueous EtOH or dilute H2SO4 below 39o when it forms the heptahydrate, and between 39o and 70o it forms the hexahydrate, and above 70o the monohydrate is stable. The anhydrous salt is obtained from the hydrates by heating at 280o or lower temperatures in a current of dry air. It decomposes to ZnO and SO2 at 767o. The solubility of the heptahydrate in H2O is 5.88% at 0o, 61.92% at 30o, 66.61% at 35o and 70.05% at 39o.
황산아연 준비 용품 및 원자재
원자재
준비 용품