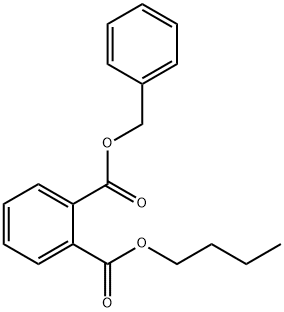
자료없음
|
|
자료없음 속성
- 녹는점
- <-35°C
- 끓는 점
- 370°C
- 밀도
- 1.1 g/mL at 25 °C(lit.)
- 증기 밀도
- 10.8 (vs air)
- 증기압
- 0.16 mm Hg ( 150 °C)
- 굴절률
- n
20/D 1.54(lit.)
- 인화점
- >230 °F
- 저장 조건
- room temp
- 용해도
- DMSO: 100 mg/mL (320.14 mM)
- 물리적 상태
- 기름진 액체
- 색상
- 투명한
- Specific Gravity
- 1.1
- 수용성
- 0.000269g/100mL
- 어는점
- -35℃
- BRN
- 2062204
- Henry's Law Constant
- (x 10-6 atm?m3/mol): 1.3 at 25 °C (calculated, Howard, 1989)
- InChIKey
- IRIAEXORFWYRCZ-UHFFFAOYSA-N
- LogP
- 4.84 at 20℃
- CAS 데이터베이스
- 85-68-7(CAS DataBase Reference)
- IARC
- 3 (Vol. Sup 7, 73) 1999
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 61-50/53-62 | ||
| 안전지침서 | 53-45-60-61 | ||
| 유엔번호(UN No.) | UN 3082 9/PG 3 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | TH9990000 | ||
| 자연 발화 온도 | 450 °F | ||
| TSCA | Yes | ||
| 위험 등급 | 9 | ||
| 포장분류 | III | ||
| HS 번호 | 29173400 | ||
| 유해 물질 데이터 | 85-68-7(Hazardous Substances Data) | ||
| 독성 | Acute oral LD50 for guinea pigs 13,750 mg/kg, mice 4,170 mg/kg, rats 2,330 mg/kg (quoted, RTECS, 1985). | ||
| 기존화학 물질 | KE-02200 | ||
| 유해화학물질 필터링 | 2006-1-558 | ||
| 중점관리물질 필터링 | 별표1-33 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 부틸벤질 프탈레이트 및 이를 25% 이상 함유한 혼합물 |
자료없음 C화학적 특성, 용도, 생산
화학적 성질
Benzyl butyl phthalate is a clear, oily liquid with a slight odor.용도
A phthalate metabolite with genotoxic effect.생산 방법
BBP is manufactured by the sequential addition of butanol and benzyl chloride to phthalic anhydride. It is used as a plasticizer for polyvinyl chloride plastics, particularly vinyl floor tile, vinyl leather, and cloth coating.일반 설명
A clear colorless liquid with a mild odor. Primary hazard is to the environment. Immediate steps should be taken to limit spread to the environment. Easily penetrates the soil to contaminate groundwater and nearby waterways.공기와 물의 반응
Slightly soluble in water and slightly denser than water.반응 프로필
Benzyl butyl phthalate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Can generate electrostatic charges. [Handling Chemicals Safely 1980. p. 250].건강위험
Prolonged contact with liquid causes some irritation of eyes and skin.화재위험
Special Hazards of Combustion Products: Irritating vapors of unburned chemical may form in fires.잠재적 노출
Benzyl butyl phthalate is used as a plasticizer for polyvinyl and cellulosic resins. It is also used as an organic intermediate. Incompatibilities: Incompatible with strong acids; nitrates, oxidizers. Destructive to rubber and paint.Carcinogenicity
The NTP examined the carcinogenicity of BBP in rats and mice. Groups of 50 male and female rats and mice were exposed to BBP via the diet at levels of 0, 6000, or 12,000 ppm (0, 300, and 600 mg/kg for rats and 0, 780, or 1560 mg/kg for mice). Male and female mice and female rats were exposed for 103 weeks. Due to poor survival, all males were sacrificed at weeks 29–30; this part of the study was later repeated. No treatmentrelated neoplasms were observed in mice. Survival was not affected. A dose-dependent reduction in body weight in both sexes was the only treatment-related effect observed in this study. Furthermore, nonneoplastic changes were all within the normal limits of incidence for B6C3F1 mice. The NTP concluded that under the conditions of the bioassay, BBP “was not carcinogenic for B6C3F1 mice of either sex.” An increased incidence of mononuclear cell leukemias was observed in the high-dose female rats. No other treatmentrelated findings were observed. The NTP concluded that BBP was “probably carcinogenic for female F344/N rats, causing an increased incidence of mononuclear cell leukemias”. The biological significance of this finding is uncertain as the background incidence of this tumor type in F344 rats is quite high.환경귀착
Biological. In anaerobic sludge diluted to 10%, benzyl butyl phthalate biodegraded to monobutyl phthalate, which subsequently degraded to phthalic acid. After 40 d, >90% of applied amount degraded (Shelton et al., 1984). When benzyl butyl phthalate (5 and 10 mg/L) was statically incubated in the dark at 25 °C with yeast extract and settled domestic wastewater inoculum, complete biodegradation with rapid adaptation was observed after 7 d (Tabak et al., 1981). In activated sludge, the half-life was 2 h (Saeger and Tucker, 1976). Gledhill et al. (1980) reported half-lives of 2 and <4 d for benzyl butyl phthalate in river water and a lake water microcosm, respectively. Aerobic degradation of benzyl butyl phthalate by acclimated soil and activated sewage sludge microbes was studied using an acclimated shake flask CO2 evolution test. After 28 d, loss of benzyl butyl phthalate (primary degradation) was 43%, with a lag phase of 15.6 d, and ultimate biodegradation (CO2 evolution) was 43%. The half-life under these conditions was 19.4 d (Sugatt et al., 1984).Surface Water. The biological half-life of benzyl butyl phthalate in river water was determined to be 2 d (Saeger and Tucker, 1976).
Photolytic. Gledhill et al. (1980) reported the photolytic half-life is >100 d.
Chemical/Physical. Benzyl butyl phthalate initially hydrolyzes to butyl hydrogen phthalate. This compound undergoes additional hydrolysis yielding o-phthalic acid, 1-butanol, and benzyl alcohol (Kollig, 1993). Gledhill et al. (1980) reported the hydrolysis half-life is >100 d.
운송 방법
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9—Miscellaneous hazardous material, Technical Name Required폐기물 처리
Atomize into an incinerator together with a flammable solvent.자료없음 준비 용품 및 원자재
원자재
준비 용품
자료없음 공급 업체
글로벌( 327)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7786 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 |
admin@hbouhuang.com | China | 1696 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1807 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1011 | 58 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |