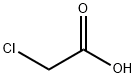
클로로아세트산
|
|
클로로아세트산 속성
- 녹는점
- 60-63 °C (lit.)
- 끓는 점
- 189 °C (lit.)
- 밀도
- 1.58
- 증기 밀도
- 3.26 (vs air)
- 증기압
- 0.75 mm Hg ( 20 °C)
- 굴절률
- 1.4330
- 인화점
- 126°C
- 저장 조건
- Store below +30°C.
- 용해도
- 메탄올, 아세톤, 디에틸 에테르, 벤젠, 클로로포름 및 에탄올에 용해됩니다.
- 산도 계수 (pKa)
- 2.85(at 25℃)
- 물리적 상태
- 액체
- 색상
- 하얀색
- 냄새
- 관통하는 타는 냄새
- pH 범위
- < 1 at 800 g/l at 20 °C
- 폭발한계
- 8%
- 수용성
- 녹는
- 감도
- Hygroscopic
- Merck
- 14,2112
- Specific Activity
- 5-10 Ci/mmol
- Solvent
- Sterile water in sealed ampoule
- Concentration
- 1 mCi/ml
- BRN
- 605438
- Dielectric constant
- 12.3(60℃)
- 안정성
- 안정적인.강염기, 알칼리, 대부분의 일반적인 금속, 강산화제와 호환되지 않습니다.
- LogP
- 0.49 at 20℃
- CAS 데이터베이스
- 79-11-8(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,N,Xi,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 25-34-50-40-36/37/38-23/24/25-38 | ||
| 안전지침서 | 23-37-45-61-36-26-16-63-36/37/39 | ||
| 유엔번호(UN No.) | UN 1751 6.1/PG 2 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | AF8575000 | ||
| F 고인화성물질 | 3 | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | II | ||
| HS 번호 | 29154000 | ||
| 유해 물질 데이터 | 79-11-8(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-05492 | ||
| 유해화학물질 필터링 | 97-1-278 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 클로로아세트산 및 이를 25% 이상 함유한 혼합물 |
클로로아세트산 C화학적 특성, 용도, 생산
개요
Chloroacetic acid (CAA) is a monohalogenated acetic acid (m-HAA) that is used as a photosensitizing agent and in industrial synthesis of certain organic chemicals such as indigoid dyes. The m-HAAs are a major class of drinking water disinfection by-products during chlorination of drinking water.화학적 성질
Chloroacetic acid is a colorless to white crystalline solid. It has a strong vinegar-like odor and an Odor Threshold of 0.15 milligram per cubic meter.용도
Chloroacetic acid behaves as a very strong monobasic acid and is used as a strong acid catalyst for diverse reactions. The Cl function can be displaced in base-catalyzed reactions.정의
A colorless crystalline solid made by substituting one of the hydrogen atoms of the methyl group of ethanoic acid with chlorine, using red phosphorus. It is a stronger acid than ethanoic acid because of the electron-withdrawing effect of the chlorine atom. Dichloroethanoic acid (dichloroacetic acid, CHCl2COOH) and trichloroethanoic acid (trichloroacetic acid,CCl3COOH) are made in the same way. The acid strength increases with the number of chlorine atoms present.생산 방법
Chloroacetic acid can be synthesized by the radical chlorination of acetic acid, treatment of trichloroethylene with concentrated H2SO4, oxidation of 1,2-dichloroethane or chloroacetaldehyde, amine displacement from glycine, or chlorination of ketene.일반 설명
Chloroacetic acid, solution is a colorless solution of the white crystalline solid. The acid concentration can be up to 80%.It is used in manufacturing dyes and in medicine. Chloroacetic acid is toxic by inhalation, ingestion and skin contact. Chloroacetic acid is corrosive to metals and tissue. Chloroacetic acid is used as an herbicide, preservative and bacteriostat.공기와 물의 반응
Water soluble.위험도
Use in foods prohibited by FDA. Irritating and corrosive to skin. Upper respiratory tract irritant. Questionable carcinogen.건강위험
Inhalation causes mucous membrane irritation. Contact with liquid causes severe irritation and burns of the eyes and irritation and burns of skin. Ingestion causes burns of mouth and stomach.화재위험
Special Hazards of Combustion Products: Toxic gases, such as hydrogen chloride, phosgene and carbon monoxide, may be generated.Safety Profile
Poison by ingestion, inhalation, subcutaneous, and intravenous routes. A corrosive skin, eye, and mucous membrane irritant. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Combustible liquid when exposed to heat or flame. To fight fire, use water spray, fog, mist, dry chemical, foam. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORIDES.잠재적 노출
This haloacetic acid can be a byproduct of drinking water disinfection and may increase the risk of cancer. Monochloracetic acid is used primarily as a chemical intermediate in the synthesis of sodium carboxymethyl cellulose; and such other diverse substances as ethyl chloroacetate, glycine, synthetic caffeine, sarcosine, thioglycolic acid, and various dyes. Hence, workers in these areas are affected. It is also used as an herbicide. Therefore, formulators and applicators of such herbicides are affected.환경귀착
CCA by inhibition of the pyruvate-dehydrogenase, aconitase, and a-ketoglutarate dehydrogenase that contribute in tricarboxylic acid cycle and also inhibition of glyceraldehyde- 3-phosphate dehydrogenase can impair production of cellular energy and conversion to anaerobic glycolysis, resulting in increasing acidosis with accumulation of glycolic acid, oxalate, and lactate production. CCA can also affect cellular components via sulfhydryl groups. Both of these effects may contribute to central nervous system (CNS), cardiovascular, renal, and hepatic effects. The metabolites glycolic acid and oxalate may contribute to CNS and renal toxicity (myoglobin and oxalate precipitation in the tubuli). Binding of calcium to oxalates probably causes the hypocalcemia, but hypocalcemia can be secondary to rhabdomyolysis. CAA by reduction of cellular glutathione can cause oxidative stress. Inhibition of mitochondrial aconitase causes hypoglycemia.운송 방법
UN1750 (liquid) & UN1751 (solid) Chloroacetic acid, solid or liquid, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 8-Corrosive material.Purification Methods
Crystallise the acid from CHCl3, CCl4, *benzene or water. Dry it over P2O5 or conc H2SO4 in a vacuum desiccator. Further purification is by distillation from MgSO4, and by fractional crystallisation from the melt. Store it under vacuum or under dry N2. [Bernasconi et al. J Am Chem Soc 107 3621 1985, Beilstein 2 IV 474.]비 호환성
Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water, and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, sulfides (releasing heat, toxic, and possibly flammable gases), thiosulfates, and dithionites (releasing hydrogen sulfate and oxides of sulfur). The solution in water is a strong acid. Contact with strong oxidizers, strong bases; and strong reducing agents such as hydrides can cause violent reactions. Chloracetic acid decomposes on heating, producing toxic and corrosive hydrogen chloride, phosgene, and carbon monoxide gases. Attacks metals in the presence of moisture.폐기물 처리
Incineration, preferably after mixing with another combustible fuel; care must be exercised to assure complete combustion to prevent the formation of phosgene; an acid scrubber is necessary to remove the halo acids produced.클로로아세트산 준비 용품 및 원자재
원자재
유황
일염화유황
염소(기체)
디클로로아세트산메틸에스테르
트라이클로로에틸렌
디클로로아세트산
에틸렌 클로로히드린
염산
아세트산(빙초산)
1,1,2,2-테트라클로로에틸렌
글리콜산
트리클로르아세트 산
준비 용품
디이소프로필말로네이트
[(2-OXO-2H-CHROMEN-7-YL)OXY]ACETIC ACID
4-클로로-o-톨옥시 아세트산
1-NAPHTHOXYACETIC ACID
(2,4-디클로로페녹시)아세트산 다이메틸아민
2-퀴노잘리놀
(1-METHYL-1H-BENZIMIDAZOL-2-YL)METHYLAMINE
Methoxyacetyl chloride
Propacetamol
에틸렌디아민테트라아세트산, 테트라나트륨 염
페녹시초산
2-Hydrazinyl-N,N-dimethylacetamide
요오드아세트산
2-(CARBOXYMETHYLTHIO)-4,6-DIMETHYLPYRIMIDINE MONOHYDRATE
(METHYLTHIO)ACETIC ACID
2-Hydrazinylacetamide
디클로로아세트산메틸에스테르
Betaine hydrochloride
C.I. 색소 적색 181
N-METHYL-N-PHENYL-2-PIPERAZIN-1-YLACETAMIDE
에틸렌디아민테트라아세트산, 테트라나트륨 염
sulfate AEC
Ethionine ester
ETHYL O-CARBOETHOXYMETHYLSALICYLATE
4-클로로페녹시아세트산
2-Chloro-3',4'-dihydroxyacetophenone
카보시스테인
2-Hydrazinyl-N-methylacetamide
Acetochlor E.C.
3-(2,4-difluorophenoxy)propanoic acid
2-Benzofurancarboxaldehyde
2-Nitrophenoxyacetic acid
말론 산, 디나트륨 염
2,4-디클로로페녹시에타노익 엑시드
Synthetic greasing agent
에틸말론산디에칠에스테르
instant soluble Tian-jing gum
나이트릴로트라이아세트산
히드록시아세트산, 나트륨 염
2,4-Dichlorophenoxybutyric acid