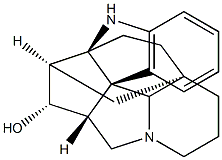
(2R,3R,5R,11S,22S)-3,11-Methanoaspidofractinin-22-ol
|
|
(2R,3R,5R,11S,22S)-3,11-Methanoaspidofractinin-22-ol 속성
- 녹는점
- 239-245°C (dec.).
안전
(2R,3R,5R,11S,22S)-3,11-Methanoaspidofractinin-22-ol C화학적 특성, 용도, 생산
개요
An imidazolidinocarbazole alkaloid, this base occurs in Aspidosperma duckei Hub. and also in A. macrocarpon Mart. It may be purified by recrystallization from either MeOH or CHC1 3 when it forms colourless needles. It has [α]27D + 16.7° (c 0.6, CHC13 ). The ultraviolet spectrum in ethanol solution has absorp-tion maxima at 246 and 297 mil. In 5N/HCl the absorption maxima occur at 262 and 268.5 mil with shoulders at 249 and 255 mil. The alkaloid contains one hydroxyl group and on acetylation yields the O-acetate, m.p. 202-5°C; [α]26D + 37° (c 2.97, CHCI3 ).참고 문헌
Filho et al., J. Chem. Soc., C, 1260 (1966)(2R,3R,5R,11S,22S)-3,11-Methanoaspidofractinin-22-ol 준비 용품 및 원자재
원자재
준비 용품
(2R,3R,5R,11S,22S)-3,11-Methanoaspidofractinin-22-ol 공급 업체
글로벌( 0)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|