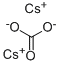
세슘 탄소산
|
|
세슘 탄소산 속성
- 녹는점
- 610 °C (dec.) (lit.)
- 밀도
- 4.072
- 증기압
- 0Pa at 25℃
- 저장 조건
- Inert atmosphere,Room Temperature
- 용해도
- 물에 용해됨
- 물리적 상태
- 분말/과립
- 색상
- 하얀색
- Specific Gravity
- 4.072
- 수용성
- 261g/100mL (20 ºC)
- 감도
- Hygroscopic
- Merck
- 14,2010
- BRN
- 4546405
- 안정성
- 안정적인. 매우 조해성. 강한 산화제, 강산과 호환되지 않습니다.
- InChIKey
- FJDQFPXHSGXQBY-UHFFFAOYSA-L
- LogP
- 0 at 20℃
- CAS 데이터베이스
- 534-17-8(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 68-36/37/38 | ||
| 안전지침서 | 22-24/25-36/37/39-26-27 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | FK9400000 | ||
| F 고인화성물질 | 3-10 | ||
| TSCA | Yes | ||
| HS 번호 | 28369918 | ||
| 기존화학 물질 | KE-05432 |
세슘 탄소산 C화학적 특성, 용도, 생산
물성
610℃-dec。물성
고체(흰색)。물성
610℃-dec。개요
Cesium carbonate is a basic carbonate and is used in organic synthesis as a mild inorganic base. It can be used in C, N, O alkylation and arylation reactions. Cesium carbonate not only promotes the carbonylation of alcohols and carbamination of amines but also inhibits side reactions common in other processes. It is also used in six-membered annulation, intramolecular and intermolecular cyclizations, Suzuki coupling, aza-Henry, nucleophilic substitution, cross-coupling and different cycloaddition reactions.화학적 성질
Colourless crystals or white powder , easily soluble in water, quickly absorbs moisture when placed in the air. The aqueous solution of cesium carbonate is strongly alkaline and can react with acid to produce the corresponding cesium salt and water, and release carbon dioxide.용도
Cesium carbonate is a versatile reagent for organic synthesis. This inorganic base has been employed in numerous organic applications ranging from N-protection of amino acids to a base in either the Horner- Wadsworth-Emmons reaction or in Suzuki couplings. It has also found use as a catalyst for ethylene oxide polymerization, in coating for spatter-free welding of steel in CO2, as a functional interlayer in photovoltaic devices and in oxide cathodes. Cesium carbonate is also used in the beer-brewing industry to make the "head" of beer foamier.주요 응용
Cesium carbonate is widely utilized as a precursor for other cesium compounds. It acts as a base in sensitive organic reactions. It can be used as a base in C-C and C-N cross-coupling reactions such as Suzuki?Miyaura, Heck, and Buchwald-Hartwig amination reactions. It finds use in solar cells as it increases the power conversion efficiency of cells through the transfer of electrons. It is also used in the production of special optical glasses, petroleum catalytic additives, special ceramics and in the sulfuric acid industry. It is useful in the N-alkylation (of sulfonamides, beta-lactams, indoles, heterocycles and several sensitive nitrogen compounds), carbamination of amines, carbonylation of alcohols and aerobic oxidation of alcohols into carbonyl compounds without polymeric by products. Promotes the efficient O-alkylation of alcohols to form mixed alkyl carbonates.제조 방법
cesium carbonate can be prepared by thermal decomposition of caesium oxalate. Upon heating, caesium oxalate is converted to caesium carbonate with emission of carbon monoxide.Cs2C2O4 → Cs2CO3 + CO
It can also be synthesized by reacting caesium hydroxide with carbon dioxide.
2 CsOH + CO2 → Cs2CO3 + H2O
화학 반응
Many of the properties of cesium carbonate are due to the softness of the cesium cation. This softness makes cesium carbonate rather soluble in organic solvents such as alcohols, DMF and Et2O. This has rendered cesium carbonate useful in palladium chemistry, which is often carried out in non-aqueous media where insolubility of inorganic bases can limit reactivity. Cs2CO3 has, for example, been used with good results in Heck, Suzuki and Sonogashira reactions. Cesium carbonate has also received much attention for its use in O-alkylations, particularly of phenols.It has been postulated that O-alkylations of phenols using Cs2CO3 in non-aqueous solvents occurs via the ‘naked’ phenolate anion, which behaves as a strong nucleophile. Therefore, this methodology can even be applied to secondary halides, minimizing the usual unwanted side reactions such as elimination and decomposition.일반 설명
Cesium carbonate is a powerful inorganic base widely used in organic synthesis. It is a potential chemoselective catalyst for the reduction of aldehydes and ketones to alcohols. It is employed as base for the Heck coupling reaction of 4-trifluoromethyl-1-chlorobenzene and aryl chlorides.위험도
Cesium carbonate is a danger compound.H315: Causes skin irritation
H318: Causes serious eye damage
H335: May cause respiratory irritation
H361: Suspected of damaging fertility or the unborn child
H373: May cause damage to organs through prolonged or repeated exposure
Safety Profile
Moderately toxic by ingestion. Mutation data reported. When heated to decomposition it emits acrid smoke and fumes. See also CESIUM.Purification Methods
Crystallise it from ethanol (10mL/g) by partial evaporation. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 988 1963.]세슘 탄소산 준비 용품 및 원자재
원자재
준비 용품
3-PYRIDIN-4-YL-BENZOIC ACID
ETHYL 3-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOLE-4-CARBOXYLATE
3-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOLE-4-CARBOXYLIC ACID
6-IODO-5-METHOXY-2-PYRIDINECARBOXYLIC ACID
4-[(3,4-DICHLOROBENZYL)OXY]BENZALDEHYDE
1-(1H-pyrazol-1-yl)propan-2-amine
4-[(2-CHLORO-6-FLUOROBENZYL)OXY]-3-METHOXYBENZALDEHYDE
5-페닐티오펜-2-카르복살디하이드
3-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOL-4-AMINE
1-Phenylimidazole
4-(2-CHLORO-6-FLUOROBENZYLOXY)BENZALDEHYDE
4-BROMO-3-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOLE
3-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOLE-4-CARBOXAMIDE
(3-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOL-4-YL)METHANOL
2-Chloroquinoline
1-PHENYL-3-(TRIFLUOROMETHYL)PYRAZOLE
3-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOLE-4-CARBONITRILE
5-Phenylthiophene-2-carboxylic acid
3-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOLE-5-CARBOXYLIC ACID
(3-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOL-4-YL)METHANAMINE
4-CHLOROPHENOXYACETYL CHLORIDE
3-Morpholinobenzaldehyde
1-(4-Pyridyl)piperazine
BOC-3-IODO-D-ALANINE BENZYL ESTER
2-[(2,6-DICHLOROBENZYL)OXY]-5-NITROBENZALDEHYDE
3-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOLE-4-CARBALDEHYDE
3-(4-METHYLPHENYL)BENZALDEHYDE
세슘 탄소산 공급 업체
글로벌( 692)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Chemicalsolution | +86-13661650151 |
syuzwx@126.com | China | 4 | 58 |
| CHANGZHOU HARVECHEM LTD | +86-0519-88784888 +8613906120163 |
inquiry@harvechem.com | China | 245 | 58 |
| Shanghai Huaran Industrial Co.,Ltd | 17749774353 |
1715438216@qq.com | China | 52 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12453 | 58 |
| Hunan aslsen technology co.,ltd | +8615308460054 |
aslsc@aslsen.com | China | 124 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 |
sales@myuchem.com | China | 6711 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7377 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 |
sales03@chemcn.cn | China | 951 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 |
admin@hbouhuang.com | China | 343 | 58 |
| Springchem New Material Technology Co.,Limited | +86-021-62885108 +8613917661608 |
info@spring-chem.com | China | 2068 | 57 |