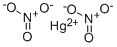질산수은(Ⅱ)(고체) C화학적 특성, 용도, 생산
화학적 성질
Mercuric nitrate is a white to yellowish crystalline solid with an odor like nitric acid. Normally exists
as the hemihydrate or the dihydrate
용도
Nitration of aromatic organic compounds, felt
manufacture, mercury fulminate manufacturing.
일반 설명
A white crystalline solid. Toxic by inhalation, ingestion and/or skin contact. Prolonged exposure to fire or heat may result in an explosion. Produces toxic oxides of nitrogen when heated to decomposition. Used to make other chemicals and in medicine.
공기와 물의 반응
Deliquescent. Soluble in a small amount of water. With much water or on boiling with water, an insoluble basic salt is formed.
반응 프로필
MERCURIC NITRATE is noncombustible, but, as an oxidizing agent, will accelerate the burning of combustible materials. If large quantities are involved in a fire or the combustible material is finely divided, an explosion may result. Light sensitive. Mixtures with alkyl esters may explode, owing to the formation of alkyl nitrates. Mixtures with phosphorus, tin(II) chloride, or other reducing agents may react explosively [Bretherick 1979. p. 108-109]. Acetylene forms a sensitive acetylide when passed into an aqueous solution of MERCURIC NITRATE [Mellor 4:933. 1946-47]. Should not be mixed with alcohols as explosive mercury fulminates may be formed [Bahme 1961. p. 9]. Is violently reduced by hypophosphoric acid [Mellor 4:993. 1946-47]. Reacts with phosphine to give a yellow precipitate that explodes when heated or subjected to shock [Mellor 4:993. 1946-47].
위험도
Dangerous fire risk in contact with organic
materials. Very toxic.
건강위험
Acute systemic poisoning may be fatal within a few minutes; death by uremic poisoning is usually delayed 5-12 days. Acute poisoning has resulted from inhaling dust concentrations of 1.2-8.5 mg/m 3 of air; symptoms inc lude tightness and pain in chest, coughing, and difficulty in breathing. Ingestion causes necrosis, pain, vomiting, and severe purging. Contact with eyes causes ulceration of conjunctiva and cornea. Contact with skin causes irritation and po ssible dermatitis; systemic poisoning can occur by absorption through skin.
Safety Profile
Poison by ingestion,
skin contact, intraperitoneal, and
subcutaneous routes. A powerful oxidizer.
Probably an eye, skin, and mucous
membrane irritant. Reacts with acetylene to form the explosive mercury acetylide whch
is sensitive to heat, friction, or contact with
sulfuric acid. Reaction with ethanol forms
the explosive mercury fulrmnate. Reaction
with isobutene forms an unstable explosive
product. Forms explosive mixtures with
phosphine (heatand impact-sensitive),
potassium cyanide (heat-sensitive), and
sulfur. Violent reaction with phosphinic
acid, hypophosphoric acid, unsaturated
hydrocarbons, aromatics. Vigorous reaction
with petroleum hydrocarbons. When heated
to decomposition it emits very toxic fumes
of Hg and NOx. See also MERCURY
COMPOUNDS, INORGANIC; and
NITRATES.
잠재적 노출
Mercuric nitrate is used in making
other chemicals; in felt manufacture and in making mercury
fulminate
운송 방법
UN1625 Mercuric nitrate, Hazard Class: 6.1;
Labels: 6.1-Poisonous materials.
비 호환성
A strong oxidizer. Reacts violently with
combustibles, petroleum hydrocarbons; reducing agents;
aldehydes, ammonia, ketones, phosphorus. Reacts with
acetylene, alcohol, phosphine, and sulfur to form shocksensitive compounds. Aqueous solution attacks most
metals. Vigorous and dangerous reaction with petroleum
hydrocarbons. Incompatible with organic materials;
acetylene, ethanol, phosphine, sulfur, hypophosphoric acid.
Inorganic mercury compounds are incompatible with acetylene, ammonia, chlorine dioxide; azides, calcium (amalgam
formation), sodium carbide; lithium, rubidium, copper.
Decomposes in heat or on exposure to light, producing
toxic fumes (mercury, nitrogen oxides)
질산수은(Ⅱ)(고체) 준비 용품 및 원자재
원자재
준비 용품