벤질 알코올
|
|
벤질 알코올 속성
- 녹는점
- -15 °C
- 끓는 점
- 205 °C
- 밀도
- 1.045 g/mL at 25 °C(lit.)
- 증기 밀도
- 3.7 (vs air)
- 증기압
- 13.3 mm Hg ( 100 °C)
- 굴절률
- n
20/D 1.539(lit.)
- 인화점
- 201 °F
- 저장 조건
- Store at +2°C to +25°C.
- 용해도
- H2O: 33 mg/mL, 투명, 무색
- 산도 계수 (pKa)
- 14.36±0.10(Predicted)
- 물리적 상태
- 액체
- 색상
- APHA: ≤20
- 상대극성
- 0.608
- 냄새
- 부드럽고 편안한 냄새
- 폭발한계
- 1.3-13%(V)
- ?? ??
- 꽃향기
- 수용성
- 4.29g/100mL(20℃)
- Merck
- 14,1124
- JECFA Number
- 25
- BRN
- 878307
- Henry's Law Constant
- <2.70 x 10-7 at 25 °C (thermodynamic method-GC/UV, Altschuh et al., 1999)
- 노출 한도
- No exposure limit is set. Because of its low vapor pressure and low toxicity, the health hazard to humans from occupational exposure should be very low.
- Dielectric constant
- 13.1(20℃)
- InChIKey
- WVDDGKGOMKODPV-UHFFFAOYSA-N
- LogP
- 1.05 at 20℃
- CAS 데이터베이스
- 100-51-6(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 20/22-63-43-36/37/38-23/24/25-45-40 | ||
| 안전지침서 | 26-36/37-24/25-23-53 | ||
| 유엔번호(UN No.) | UN 1593 6.1/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | DN3150000 | ||
| F 고인화성물질 | 8-10-23-35 | ||
| 자연 발화 온도 | 817 °F | ||
| TSCA | Yes | ||
| HS 번호 | 29062100 | ||
| 유해 물질 데이터 | 100-51-6(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 3.1 g/kg (Smyth) | ||
| 기존화학 물질 | KE-02570 |
벤질 알코올 C화학적 특성, 용도, 생산
용도
착향료로서 과자에 쓰인다. 특히 딸기나 편도의 향기에 배합한다.용도
아이스크림(160ppm), 츄잉껌(1,200ppm), 크림향료(재스민향 160ppm), 사탕, 젤라틴·푸딩(20~40ppm) 등에 이용된다. 용해력이 높기 때문에 유성향료의 용해제 및 추출제, 휘발보류제로 사용되며, 빛이 차단된 밀봉용기에 담아 보존한다. 화장품·비누용의 향료로, 재스민 등 인조 화정유의 조합, 용제나 회석제, 에스터 원료로서도 사용한다. 또 국소마취제로도 쓰이는데, 농도 1∼3%의 것은 주사제의 조정에 쓰이고, 1∼2% 첨가한 것을 로션으로 만들어 가려움을 멈추게 하는 약으로 사용한다.순도시험
(1) 비중 : 이 품목의 비중은 1.042~1.047이어야 한다.
(2) 굴절률 : 이 품목의 굴절률 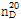 은 1.539~1.541이어야 한다.
은 1.539~1.541이어야 한다.
(3) 용상 : 이 품목 1mL를 물 50mL에 녹일 때, 탁하여도 유분을 곧 분리하여서는 아니 된다.
(4) 염소화합물 : 이 품목은 향료시험법 중 할로겐시험법의 동망법에 따라 시험할 때, 이에 적합하여야 한다.
(5) 유리산 및 유리알칼리 : 이 품목 100mL를 중화알콜 10mL에 녹여 페놀프탈레인시액 2방울을 가할 때, 홍색을 나타내어서는 아니 된다. 또 이에 0.1N 수산화나트륨용액 0.2mL를 가하여 흔들어 섞을 때, 홍색을 나타내어야 한다.
(6) 알데히드류 : 이 품목 5g을 정밀히 달아 향료시험법 중 알데히드류 및 케톤류함량측정법의 히드록실아민법 제2법에 따라 정량할 때 0.5N 염산의 소비량은 0.2mL 이하이어야 한다.
확인시험
이 품목 2~3방울을 과망간산칼륨용액(1→20) 5mL에 가하고 이에 묽은황산을 가하여 산성으로 하면 벤즈알데히드의 향기가 발생한다.
정량법
이 품목 약 0.5g을 정밀히 달아 향료시험법 중 알콜류함량측정법 제2법에 따라 시험한다.
0.5N 알콜성수산화칼륨용액 1mL = 54.07mg C7H8O
개요
Benzyl alcohol is a component catalyst for epoxy resins. It is also contained in the color developer C-22.화학적 성질
Benzyl alcohol occurs in many essential oils and foods. It is a colorless liquid with a weak, slightly sweet odor. Benzyl alcohol can be oxidized to benzaldehyde, for example, with nitric acid. Dehydrogenation over a copper–magnesium oxide–pumice catalyst also leads to the aldehyde. Esterification of benzyl alcohol results in a number of important fragrance and flavor materials. Diphenylmethane is prepared by a Friedel–Crafts reaction of benzyl alcohol and benzene with aluminum chloride or concentrated sulfuric acid. By heating benzyl alcohol in the presence of strong acids or strong bases, dibenzyl ether is formed.물리적 성질
Colorless, hygroscopic, air sensitive liquid with a faint, pleasant, aromatic odor. Odor threshold concentration in water is 10 ppm (Buttery et al., 1988).출처
The free alcohol is often present in several essential oils and extracts of jasmine, tobacco, tea, neroli, copaiba, Acacia farnesiana Willd., Acacia cavenia Hook. and Arn., Robinia pseudacacia, ylang-ylang, Pandanus odoratissimus, Michelia champaca, Prunus laurocerasus, tuberose, orris, castoreum, violet leaves, clove buds and others. Also found in fresh apple, apricot, mandarin peel oil, high bush blueberry, raspberry, strawberry fruit, American cranberry and cooked asparagus.역사
LIEBIG and WO¨HLER first prepared benzyl alcohol from bitter almond oil (benzaldehyde) in 1832. The structure of benzyl alcohol was determined in 1853 by CANNIZZARO. CANNIZZARO used the reaction named after him, in which benzaldehyde is disproportionated into benzoic acid and benzyl alcohol through the action of an alkali.용도
benzyl alcohol is a preservative against bacteria, used in concentrations of 1 to 3 percent. It can cause skin irritation.정의
ChEBI: Benzyl alcohol is an aromatic alcohol that consists of benzene bearing a single hydroxymethyl substituent. It has a role as a solvent, a metabolite, an antioxidant and a fragrance.제조 방법
Benzyl alcohol is prepared commercially by the distillation of benzyl chloride with potassium or sodium carbonate. It may also be prepared by the Cannizzaro reaction of benzaldehyde and potassium hydroxide.World Health Organization (WHO)
Benzyl alcohol has been used as an antimicrobial agent in pharmaceutical preparations for many years. Parenteral administration of preparations containing 0.9% benzyl alcohol resulted in the death of 16 neonates in the USA in the early 1980s. Many countries subsequently warned against using such preparations in neonates. This decision is not applicable to the use of benzyl alcohol as a preservative in other circumstances or to its use in topical preparations and no country has placed a total ban on the compound.일반 설명
A clear colorless liquid with a pleasant odor. Slightly denser than water. Flash point 194°F. Boiling point 401°F. Contact may irritate skin, eyes, and mucous membranes. May be slightly toxic by ingestion. Used to make other chemicals.공기와 물의 반응
Slightly soluble in water.반응 프로필
Attacks plastics. [Handling Chemicals Safely 1980. p. 236]. Acetyl bromide reacts violently with alcohols or water [Merck 11th ed. 1989]. Mixtures of alcohols with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions. Example: an explosion will occur if dimethylbenzylcarbinol is added to 90% hydrogen peroxide then acidified with concentrated sulfuric acid. Mixtures of ethyl alcohol with concentrated hydrogen peroxide form powerful explosives. Mixtures of hydrogen peroxide and 1-phenyl-2-methyl propyl alcohol tend to explode if acidified with 70% sulfuric acid [Chem. Eng. News 45(43):73 1967; J, Org. Chem. 28:1893 1963]. Alkyl hypochlorites are violently explosive. They are readily obtained by reacting hypochlorous acid and alcohols either in aqueous solution or mixed aqueous-carbon tetrachloride solutions. Chlorine plus alcohols would similarly yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M 1991]. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence [Wischmeyer 1969].위험도
Highly toxic.건강위험
Benzyl alcohol is a low acute toxicant witha mild irritation effect on the skin. Theirritation in 24 hours from the pure compoundwas mild on rabbit skin and moderateon pig skin. A dose of 750 μg producedsevere eye irritation in rabbits. The toxicityof benzyl alcohol is of low order,the effects varying with the species. Oralintake of high concentrations of this compoundproduced behavioral effects in rats.The symptoms progressed from somnolenceand excitement to coma. Intravenous administrationin dogs produced ataxia, dyspnea,diarrhea, and hypermotility in the animals.Adult and neonatal mice treated withbenzyl alcohol exhibited behavioral change,including sedation, dyspnea, and loss ofmotor function. Pretreatment with pyrazoleincreased the toxicity of benzyl alcohol. Withdisulfiram the toxicity remained unchanged.The study indicated that the acute toxicitywas due to the alcohol itself andnot to bezaldehyde, its primary metabolite(McCloskey et al. 1986).
화재위험
Benzyl alcohol is combustible.Pharmaceutical Applications
Benzyl alcohol is an antimicrobial preservative used in cosmetics, foods, and a wide range of pharmaceutical formulations, including oral and parenteral preparations, at concentrations up to 2.0% v/v. The typical concentration used is 1% v/v, and it has been reported to be used in protein, peptide and small molecule products, although its frequency of use has fallen from 48 products in 1996, 30 products in 2001, to 15 products in 2006. In cosmetics, concentrations up to 3.0% v/v may be used as a preservative. Concentrations of 5% v/v or more are employed as a solubilizer, while a 10% v/v solution is used as a disinfectant.Benzyl alcohol 10% v/v solutions also have some local anesthetic properties, which are exploited in some parenterals, cough products, ophthalmic solutions, ointments, and dermatological aerosol sprays.
Although widely used as an antimicrobial preservative, benzyl alcohol has been associated with some fatal adverse reactions when administered to neonates. It is now recommended that parenteral products preserved with benzyl alcohol, or other antimicrobial preservatives, should not be used in newborn infants if at all possible.
색상 색인 번호
Benzyl alcohol is mainly a preservative, mostly used in topical antimycotic or corticosteroid ointments. It is also a component catalyst for epoxy resins and is contained in the color developer C-22. As a fragrance allergen, it has to be mentioned by name in cosmetics within the EU.Carcinogenicity
In an NTP study, F344 rats were dosed by oral gavage with 0, 200, and 400 mg/kg, 5 days/ week for 2 years. Benzyl alcohol had no effect on the survival of male rats; female rats had reduced survival, and many of the early deaths were considered related to the gavage procedure. There were no treatment-related effects on nonneoplastic or neoplastic lesions in either sex treated with benzyl alcohol. It was concluded that under the conditions of the study, there was no evidence of carcinogenic activity . In the same NTP study, B6C3F1 mice were dosed by oral gavage with 0, 100, and 200 mg/kg, 5 days/week for 2 years. No effects on survival or body weight gain were observed. There were no treatment-related effects on nonneoplastic or neoplastic lesions in either sex. It was concluded that under the conditions of the study, there was no evidence of carcinogenic activity.환경귀착
Biological. Heukelekian and Rand (1955) reported a 5-d BOD value of 1.55 g/g which is 61.5% of the ThOD value of 2.52 g/g.Chemical/Physical. Slowly oxidizes in air to benzaldehyde (Huntress and Mulliken, 1941). Benzyl alcohol will not hydrolyze because it has no hydrolyzable functional group (Kollig, 1993).
신진 대사
Esters of benzyl alcohol are rapidly hydrolysed in vivo to benzyl alcohol, which is then oxidized . The animal organism readily oxidizes benzyl alcohol to benzoic acid, which after conjugation with glycine is rapidly eliminated as hippuric acid in the urine.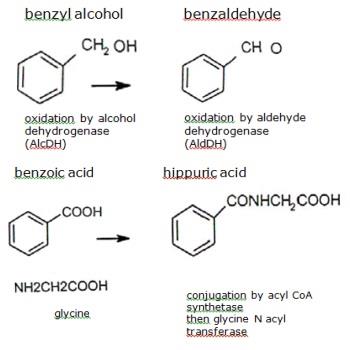
Benzyl alcohol is oxidised by alcohol dehydrogenase (AlcDH), a cytoplasmic enzyme present mainly in the liver, but also in the intestine and kidney. This reaction is saturable. The benzaldehyde formed is oxidised by aldehyde dehydrogenases (AldDH), cytoplasmic and mitochondrial enzymes mainly present in the liver, but also in the intestine and numerous organs.
저장
Benzyl alcohol oxidizes slowly in air to benzaldehyde and benzoic acid; it does not react with water. Aqueous solutions may be sterilized by filtration or autoclaving; some solutions may generate benzaldehyde during autoclaving.Benzyl alcohol may be stored in metal or glass containers. Plastic containers should not be used; exceptions to this include polypropylene containers or vessels coated with inert fluorinated polymers such as Teflon.
Benzyl alcohol should be stored in an airtight container, protected from light, in a cool, dry place.
Purification Methods
It is usually purified by careful fractional distillation under reduced pressure in the absence of air. Benzaldehyde, if present, can be detected by UV absorption at 283nm. It has also been purified by shaking with aqueous KOH and extracting with peroxide-free diethyl ether. After washing with water, the extract is treated with saturated NaHS solution, filtered, washed, dried with CaO and distilled under reduced pressure [Mathews J Am Chem Soc 48 562 1926]. Peroxy compounds can be removed by shaking with a solution of Fe2+ followed by washing the alcohol layer with distilled water and fractionally distilling it. [Beilstein 6 IV 2222.]비 호환성
Benzyl alcohol is incompatible with oxidizing agents and strong acids. It can also accelerate the autoxidation of fats. Although antimicrobial activity is reduced in the presence of nonionic surfactants, such as polysorbate 80, the reduction is less than is the case with hydroxybenzoate esters or quaternary ammonium compounds.Benzyl alcohol is incompatible with methylcellulose and is only slowly sorbed by closures composed of natural rubber, neoprene, and butyl rubber closures, the resistance of which can be enhanced by coating with fluorinated polymers. However, a 2% v/v aqueous solution in a polyethylene container, stored at 208℃, may lose up to 15% of its benzyl alcohol content in 13 weeks. Losses to polyvinyl chloride and polypropylene containers under similar conditions are usually negligible. Benzyl alcohol can damage polystyrene syringes by extracting some soluble components
Regulatory Status
Included in the FDA Inactive Ingredients Database (dental injections, oral capsules, solutions and tablets, topical, and vaginal preparations). Included in parenteral and nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.벤질 알코올 준비 용품 및 원자재
원자재
준비 용품
벤질 뷰틸산
Methyl benzenesulfonate
4-BENZYLOXY-2(1 H)-PYRIDONE
DIBENZYL DIISOPROPYLPHOSPHORAMIDITE
Benzenemethanol, ar-methyl-, acetate
CYCLOPROTHRIN
Benzyl 2-chloroacetate
5-(BENZYLOXY)-1H-PYRROLO[3,2-B]PYRIDINE-2-CARBALDEHYDE
아조벤젠
4-BENZYLOXY-5-BROMO-2-CHLOROPYRIMIDINE
5-(BENZYLOXY)PYRIDIN-3-AMINE
4-(Benzyloxy)pyridine N-oxide
3-Amino-5-hydroxypyridine
4-BENZYLOXY-2-CHLOROPYRIMIDINE
6-benzylaminopurine
디펜히드라민
GUSPERIMUS
BENZHYDRYL B-CHLOROETHYL ETHER
3-(BENZYLOXY)-5-BROMOPYRIDINE
Benzyl isobutyrate
페닐레프린 수화염화물
3-Amino-5-fluoropyridine
N-Phospho-L-arginine
BENZYL ISOVALERATE
벤질 3-페닐프로펜산
2 , 4-BIS(BENZYLOXY)-5-BROMOPYRIMIDINE
BENZYL BUTYL ETHER
프로피온산 벤질
포름산벤질에스테르
5,12-DIHYDRO-5,7,12,14-TETRAZAPENTACENE
Phenyl-methyl -hydroxybenzenebutanoate
6-BENZYLOXYPURINE
scavenger of fabric maculae
디벤질에테르
초산 벤질
벤질벤조에이트
6-O-Benzylguanine
BENZYL TIGLATE
4,6-Diamino-2-pyrimidinol
PHOSPHO-L-ARGININE SODIUM
벤질 알코올 공급 업체
글로벌( 1474)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Zaoyang ruikang chemical co ltd | +8613886234233 |
rk@zyrkhg.com | China | 1 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Jiangxi Alpha Hi-tech Pharmaceutical Co., Ltd | 510-85010237 |
overseamarketing@alphahi-tech.com | CHINA | 41 | 58 |
| Wuhan Biet Co., Ltd. | +8617320528784 |
min@biet.com.cn | China | 41 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| Hebei Linwo New Material Technology Co., LTD | +8618134091113 |
sales5@hblwxcl.com | China | 991 | 58 |
| Biopole Pharmatech Co., Ltd. | +8615151475053 |
biopole@163.com | China | 37 | 58 |
| Nanjing Deda New Material Technology Co., Ltd | +8613223293093 |
bella@njdeda.com | China | 81 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Hebei Dangtong Import and export Co LTD | +8615632927689 |
admin@hbdangtong.com | China | 991 | 58 |







