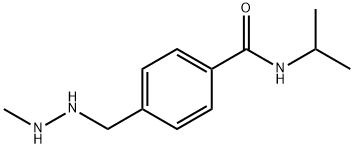
프로카바진 C화학적 특성, 용도, 생산
화학적 성질
Procarbazine is a white to pale yellow crystal-
line powder with a slight odor.
용도
antibacterial
정의
ChEBI: A benzamide obtained by formal condensation of the carboxy group of 4-[(2-methylhydrazino)methyl]benzoic acid with the amino group of isopropylamine. An antineoplastic chemotherapy drug used for treatment of Hodgkin's lymphoma. Metabolism yields azo-procar
azine and hydrogen peroxide, which results in the breaking of DNA strands.
Indications
Procarbazine (Matulane) may autooxidize spontaneously,
and during this reaction hydrogen peroxide
and hydroxyl free radicals are generated. These highly
reactive products may degrade DNA and serve as one
mechanism of procarbazine-induced cytotoxicity. Cell
toxicity also may be the result of a transmethylation reaction
that can occur between the N-methyl group of
procarbazine and the N7 position of guanine.
Mechanism of action
Procarbazine is rapidly absorbed after oral administration
and has a plasma half-life of only 10 minutes.
The drug crosses the blood-brain barrier, reaching levels
in CSF equal to those obtained in plasma.
Metabolism is extensive and complex. Urinary excretion
accounts for 70% of the procarbazine and its
metabolites lost during the first 24 hours after drug administration.
Clinical Use
When originally tested as a single agent in advanced
Hodgkin’s disease, procarbazine produced tumor regression
responses that were brief, usually lasting only 1
to 3 months. The combination of procarbazine with
mechlorethamine, vincristine, and prednisone in the
MOPP regimen, however, resulted in an 81% complete
remission rate in Hodgkin’s disease. Most of these patients
are considered cured. Procarbazine is also used in
various combination chemotherapy protocols for non-
Hodgkin’s lymphomas and small cell anaplastic (oat
cell) carcinoma of the lung. Limited antitumor effects
have been observed against multiple myeloma,
melanoma, and non–oat cell lung cancers.
부작용
The major side effects associated with procarbazine
therapy are nausea and vomiting, leukopenia, and thrombocytopenia.
Skin rashes have been reported, as have
rare cases of allergic interstitial pneumonia. Procarbazine
administration produces a high degree of chromosomal
breakage, and the compound is mutagenic, teratogenic,
and carcinogenic in experimental systems.
Procarbazine may potentiate the effects of tranquilizers
and hypnotics. Hypertensive episodes can result if
procarbazine is administered simultaneously with
adrenomimetic drugs or with tyramine-containing
foods. Rarely, a reaction to alcohol similar to that provoked
by disulfiram may occur.
잠재적 노출
Procarbazine is available in capsule
form. The primary use of this drug is as an antineoplastic
agent in the treatment of advanced Hodgkin’s disease, and
oat-cell carcinoma of the lung. The hydrochloride com-
pound is used in treatment. The FDA approved use of pro-
carbazine hydrochloride in 1969 and indicated that the drug
should be used as an adjunct to standard therapy. Possible
exposure occurs during manufacture of the drug and direct
exposure during its subsequent administration to patients.
Some of the metabolites of procarbazine hydrochloride are
both carcinostatic and carcinogenic.
Carcinogenicity
Procarbazine and procarbazine hydrochloride are reasonably anticipated to be human carcinogens based on sufficient evidence of carcinogenicity from studies in experimental animals. The names “procarbazine” and “procarbazine hydrochloride” are used interchangeably in published studies; because only procarbazine hydrochloride is produced, it has been assumed that procarbazine hydrochloride was the substance under study.
운송 방법
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required.
비 호환성
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or
explosions. Keep away from alkaline materials, strong
bases, strong acids, oxoacids, epoxides.
폐기물 처리
It is inappropriate and
possibly dangerous to the environment to dispose of
expired or waste drugs and pharmaceuticals by flushing
them down the toilet or discarding them to the trash.
Household quantities of expired or waste pharmaceuticals
may be mixed with wet cat litter or coffee grounds,
double-bagged in plastic, discard in trash. Larger quanti-
ties shall carefully take into consideration applicable
DEA, EPA, and FDA regulations. If possible return the
pharmaceutical to the manufacturer for proper disposal
being careful to properly label and securely package
the material. Alternatively, the waste pharmaceutical
shall be labeled, securely packaged and transported by a
state licensed medical waste contractor to dispose by
burial in a licensed hazardous or toxic waste landfill
or incinerator.
프로카바진 준비 용품 및 원자재
원자재
준비 용품