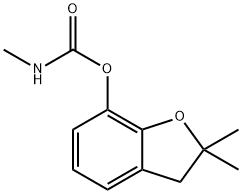
카보퓨란 C화학적 특성, 용도, 생산
개요
Carbofuran is another systemic insecticidal/nematicidal
carbamate available in granular and liquid formulations.
Because use of carbofuran granules was associated
with bird kills, the U.S. Environmental Protection
Agency (EPA) prohibited the use of carbofuran granules
in 1994.
화학적 성질
Carbofuran is a broad-spectrum carbamate insecticide and nematicide. It is an odorless,
white crystalline solid. On heating, it breaks down and can release toxic fumes, and irritating or poisonous gases. It is sparingly soluble in water, but very soluble in acetone,
acetonitrile, benzene, and cyclohexone. The liquid formulations of carbofuran are classifi ed as RUPs because of their acute oral and inhalation toxicity to humans. Granular
formulations are also classifi ed as an RUP. In fact, carbofuran was fi rst registered in the
United States in 1969 and classifi ed as an RUP. Exposure to heat breaks down carbofuran,
with the release of toxic fumes. Carbofuran is used for the control of soil-dwelling and
foliar-feeding insects. It is also used for the control of aphids, thrips, and nematodes that
attack vegetables, ornamental plants, crops of sunfl ower, potatoes, peanuts, soybeans,
sugar cane, cotton, rice, and a variety of other crops
용도
Carbofuran is used to control soil-dwelling insect pests and nematodes in a wide range of crops.
일반 설명
Carbofuran is an odorless white crystalline solid. Contact with skin may burn skin and eyes. When exposed to heat or flames Carbofuran may emit toxic oxides of nitrogen. Carbofuran is toxic by inhalation, skin contact, and/ or ingestion. Carbofuran is used as a pesticide.
공기와 물의 반응
Slightly soluble in water.
반응 프로필
Carbofuran is a carbamate ester. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates. Carbofuran is unstable in an alkaline media. .
화재위험
May release nitrogen oxides. Containers may explode in heat of fire. Avoid alkalies. Stable under neutral or acid conditions.
농업용
Insecticide, Acaricide, Nematicide: Carbofuran is a broad-spectrum carbamate pesticide that kills insects, mites, and nematodes on contact or after ingestion. It is used against soil and foliar pests of field, fruit, vegetable, and forest crops. Carbofuran, granule form, is banned in the U.S. A U.S. EPA restricted Use Pesticide (RUP). Not approved for use in EU countries. There are 40 global suppliers.. According to the Ecological Incident Investigation System, carbofuran has been responsible for more avian deaths than any other pesticide.
상품명
A13-27164®; AU'ULTRAMICIN®; BAY 704143®; BAY 78537®; BRIFUR®; CARBODAN®;
CARBOSIP 5G®; CRISFURAN®; CURETERR®; CHINUFUR®; D 1221®; DIAFURAN®; FMC 10242®; FURACARB®; FURADAN®; FURAN®; FURODAN®; KENFURAN®; KENOFURAN®; NEX®; NIA10242; NIAGARA 10242; NIAGARA NIA-10242; PILLARFURAN®; RAMPART®; YALTOX®
색상 색인 번호
It is a pesticide with insecticide properties, of the carbamate
group. It was implicated as a sensitizer in two farmers
Safety Profile
to decomposition it emits toxic fumes of
잠재적 노출
A potential danger to those involved
in the manufacture, formulation, and application of this
insecticide, acaricide, and nematocide.
환경귀착
Carbofuran is soluble in water and is moderately persistent in soil. Its half-life is 30–120 days. It enters surface water as a result of runoff from treated fields and enters groundwater by leaching of treated crops. If released to soil, degradation occurs by chemical hydrolysis and biodegradation. The persistence of carbofuran in the soil increases as the clay and organic matter content of the soil increase, and as the pH and moisture content of soil decrease. Chemical hydrolysis occurs more rapidly in alkaline soil as compared to neutral or acidic soils. Carbofuran is likely to leach to groundwater in soils with low organic content. Volatilization from soil is not expected to be significant, although some evaporation from plants may occur. If released to water, carbofuran degrades by hydrolysis under alkaline conditions and by biodegradation. Aquatic volatilization, adsorption, and bioconcentration are not expected to be important.
신진 대사 경로
The fate of carbofuran has been investigated in soils, plants, mammals,
birds, fish and insects. Metabolic pathways include hydrolysis, oxidation
(ring and N-methyl hydroxylation) and conjugation. The metabolism of
carbofuran has been extensively reviewed by Schlagbauer and Schlagbauer
(1972) and Kuhr and Dorough (1976). Metabolism in economic
animals was reviewed by Akhtar (1985). Consequently the many primary
publications are not usually cited.
신진 대사
Carbofuran (1) is degraded by hydrolysis and oxidation
in soil before ultimate mineralization . The
rate of hydrolysis in soils is slightly higher under flooded
than under nonflooded conditions. Products depend on
the soil type and the prevalence of aerobic or anaerobic
conditions, and it was also reported that carbofuran
did not degrade under anaerobic conditions. The products,
3-hydroxycarbofuran (2) and 3-ketocarbofuran (3), have
been isolated from soil extracts after incubation with carbofuran.
The phenol (7) was identified as a major product
in several studies. The products are further degraded and
bound to soil organic matter. Enhanced degradation may
follow repeated applications of carbofuran to soils, and
bacterial cultures capable of rapidly degrading carbofuran
have been obtained from treated soils.
저장
Carbofuran should be stored in a cool, dry, well-ventilated place, in their original containers only. It should not be kept stored or used near heat, open flame, or hot surfaces
운송 방법
UN2757 Carbamate pesticides, solid, toxic,
Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN
2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1;
Labels: 6.1-Poisonous materials, Technical Name RequiredUN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1;
Labels: 6.1-Poisonous materials, Technical Name Required
비 호환성
Alkaline substances, acid, strong oxidizers, such as perchlorates, peroxides, chlorates, nitrates,
permanganates.
폐기물 처리
Alkaline hydrolysis is the
recommended mode of disposal. In accordance with
40CFR165, follow recommendations for the disposal of
pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting
your local or federal environmental control agency, or by
contacting your regional EPA office.
주의 사항
During use/handling of carbofuran, workers should wear coveralls or a long-sleeved
uniform, head covering, and chemical protective gloves made of materials such as rubber, neoprene, or nitrile. Occupational workers should know that areas treated with carbofuran are hazardous. The runoff of carbofuran material and the fi re control releases
irritating or poisonous gases. It is advisable that workers should enter storehouses or
carbofuran-treated close spaces with caution
카보퓨란 준비 용품 및 원자재
원자재
준비 용품