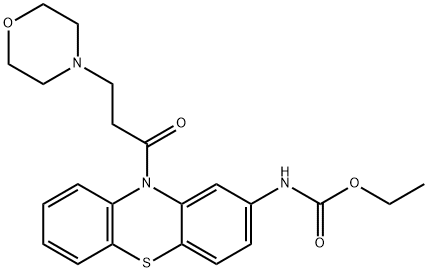
Moricizine
|
|
Moricizine 속성
- 녹는점
- 156-157°
- 끓는 점
- 625.0±55.0 °C(Predicted)
- 밀도
- 1.315±0.06 g/cm3(Predicted)
- 저장 조건
- -20°C Freezer, Under inert atmosphere
- 용해도
- 클로로포름(약간 용해됨), 메탄올(매우 소량)
- 산도 계수 (pKa)
- 6.4(at 25℃)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 옅은 오렌지색에서 밝은 오렌지색까지
안전
Moricizine C화학적 특성, 용도, 생산
용도
Moricizine, is Phenothiazine (P318040) derivative, which It was used as an Antiarrhythmic agent. It is also shown that moracizine is effective in suppressing premature ventricular contractions, couplets, and nonsustained ventricular tachycardia.정의
ChEBI: A phenothiazine substituted on the nitrogen by a 3-(morpholin-4-yl)propanoyl group, and at position 2 by an (ethoxycarbonyl)amino group.일반 설명
Moricizine, ethyl 10-(3-morpholinopropionyl)phenothiazine-2-carbamate (Ethmozine), is aphenothiazine derivative used for the treatment of malignantventricular arrhythmias. It is categorized as a class ICantiarrhythmic agent, blocking the Na+ channel with 1:1stochiometry. The drug has higher affinity for the inactivatedstate than the activated or resting states. It appearsto bind to a site on the external side of the Na channelmembrane. It has been used to suppress life-threateningventricular arrhythmias.Clinical Use
Moricizine (Ethmozine) is an antiarrhythmic used to treat documented life-threatening arrhythmias.Moricizine is indicated for the treatment of documented ventricular arrhythmias, particularly sustained ventricular tachycardia. Moricizine was evaluated in the CAST II clinical trial for the prevention of postinfarction ventricular premature complexes. It was ineffective and found to be proarrhythmic. Patients in the moricizine arm of the trial exhibited a greater incidence of sudden cardiac death than did controls.
부작용
The principal adverse gastrointestinal effect of moricizine is nausea (7%). Abdominal discomfort has also been reported. Dizziness (11%) is the most frequently reported CNS-related adverse effect. Such reactions increase in frequency with prolonged drug administration. As with other antiarrhythmic drugs, moricizine has proarrhythmic activity, which may manifest as new ventricular ectopic beats or a worsening of preexisting ventricular arrhythmias. These effects are most common in patients with depressed left ventricular function and a history of congestive heart failure. Cardiovascular effects requiring drug withdrawal include conduction defects, sinus pauses, junctional rhythm, and A-V block.Drug interactions
Clinically significant interactions with moricizine do not appear to exist.주의 사항
Patients with preexisting second- or third-degree A-V block, cardiogenic shock, or drug hypersensitivity should not be treated with moricizine.Moricizine 준비 용품 및 원자재
원자재
5-(4-Methylphenyl)-1H-tetrazole
페노티아진
Morpholine hydrochloride
우레탄
Anhydrous toluene
디에틸에테르
3-클로로프로피온산 클로라이드
황산마그네슘
ETHYL PHENTHIAZINE-2-CARBAMATE
MORPHOLINIUM CHLORIDE
준비 용품
Moricizine 공급 업체
글로벌( 56)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 19892 | 58 |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 |
info@finetechnology-ind.com | China | 9702 | 58 |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 |
gksales1@gk-bio.com | China | 9339 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 |
admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 20000 | 58 |
| Amadis Chemical Company Limited | 571-89925085 |
sales@amadischem.com | China | 131981 | 58 |
| Dandong Yichuang Pharmaceutical Co., Ltd | 0415-3124267 |
China | 35 | 60 | |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 |
jkinfo@jkchemical.com | China | 96815 | 76 |
| LGM Pharma | 1-(800)-881-8210 |
inquiries@lgmpharma.com | United States | 2127 | 70 |
Moricizine 관련 검색:
우레탄
Dronedarone
Propafenone
Antazoline
Eperisone
Moricizine
3-MORPHOLIN-4-YL-PROPIONALDEHYDE
MORACIZINE HYDROCHLORIDE
ETHYL (3-AMINOPHENYL)CARBAMATE
3-AMINO-1-PHENOTHIAZIN-10-YL-PROPAN-1-ONE
10-propionylphenothiazine
ethacizine
PHENOTHIAZINE-10-CARBOXALDEHYDE
N-(3-aminophenyl)-N-methylacetamide
ETHYL PHENOTHIAZINE-2-CARBAMATE
3-((ETHOXYCARBONYL)AMINO)DIPHENYLAMINE
n-(m-aminophenyl)aniline
Formamide,N-(3-aminophenyl)-N-methyl-