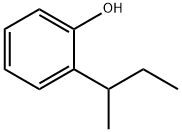
0-sec-부틸페놀 C화학적 특성, 용도, 생산
화학적 성질
colourless to light yellow liquid
용도
Chemical intermediate in preparation of resins,
plasticizers, surface-active agents.
정의
ChEBI: A member of the class of phenols that is phenol carrying a butan-2-yl group at position 2.
일반 설명
Clear colorless liquid.
공기와 물의 반응
Insoluble in water.
반응 프로필
Phenols, such as 2-sec-Butylphenol, do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid.
위험도
Skin, eye, and upper respiratory tract irri-
tant.
건강위험
o-sec-Butylphenol is a skin, eye,
and respiratory irritant.
Acute occupational exposures have resulted
in mild respiratory irritation as well as
skin burns.
화재위험
2-sec-Butylphenol is combustible.
Safety Profile
A poison by
intraperitoneal and intravenous routes.
Moderately toxic by ingestion and skin
contact. A severe skin and eye irritant.
Combustible when exposed to heat or
flame. To fight fire, use foam, spray, CO2,
dry chemical. When heated to
decomposition it emits acrid and irritating
fumes. See also PHENOL and other butyl
phenols
잠재적 노출
Butylphenols may be used as intermediates in manufacturing varnish and lacquer resins; as a
germicidal agent in detergent disinfectants; as a pour point
depressant, in motor-oil additives; de-emulsifier for oil;
soap-antioxidant, plasticizer, fumigant, and insecticide
운송 방법
UN2430 Alkylphenols, solid, n.o.s. (including
C2-C12 homologues), Hazard class: 8; Labels: 8—
Corrosive material
비 호환성
Vapors may form explosive mixture with
air. These phenol/cresol materials can react with oxidizers;
reaction may be violent. Incompatible with strong reducing
substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat
of the reaction may cause the gas to ignite and explode.
Heat is also generated by the acid-base reaction with bases;
such heating may initiate polymerization of the organic
compound. React with boranes, alkalies, aliphatic amines,
amides, nitric acid, sulfuric acid. Phenols are sulfonated
very readily (for example, by concentrated sulfuric acid at
room temperature). These reactions generate heat. Phenols
are also nitrated very rapidly, even by dilute nitric acid and
can explode when heated. Many phenols form metal salts
that may be detonated by mild shock
0-sec-부틸페놀 준비 용품 및 원자재
원자재
준비 용품