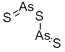비소 황화합물
|
|
비소 황화합물 속성
- 녹는점
- 300 °C(lit.)
- 끓는 점
- 707 °C
- 밀도
- 3.43 g/mL at 25 °C(lit.)
- 인화점
- 707°C
- 저장 조건
- Poison room
- 용해도
- insoluble in H2O; soluble in alkaline solutions
- 물리적 상태
- 가루
- Specific Gravity
- 3.43
- 색상
- 노란색에서 주황색으로
- 수용성
- 물에 불용성. 알칼리 황화물 또는 탄산염, 뜨거운 HCl에 용해됨
- 감도
- moisture sensitive
- Merck
- 14,806
- Solubility Product Constant (Ksp)
- pKsp: 21.68
- 노출 한도
- ACGIH: TWA 0.01 mg/m3
NIOSH: IDLH 5 mg/m3; Ceiling 0.002 mg/m3
- CAS 데이터베이스
- 1303-33-9(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 23/25-50/53 | ||
| 안전지침서 | 20/21-28-45-60-61 | ||
| 유엔번호(UN No.) | UN 1557 6.1/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | CG2638000 | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | III | ||
| HS 번호 | 2813909000 | ||
| 유해 물질 데이터 | 1303-33-9(Hazardous Substances Data) | ||
| 독성 | mouse,LD50,intraperitoneal,215mg/kg (215mg/kg),LUNGS, THORAX, OR RESPIRATION: DYSPNEABEHAVIORAL: MUSCLE WEAKNESSGASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA",Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 28(7), Pg. 53, 1984. | ||
| 기존화학 물질 | KE-01940 | ||
| 유해화학물질 필터링 | 97-1-119 | ||
| 중점관리물질 필터링 | 별표1-98 | ||
| 암, 돌연변이성물질 필터링 | 10 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 비소 또는 그 화합물과 비소화합물을 0.1% 이상 함유한 혼합물 |
비소 황화합물 C화학적 특성, 용도, 생산
화학적 성질
yellow to orange powder물리적 성질
Yellow or orange monoclinic crystal or powder; a red allotrope modification also known; density 3.46 g/cm3; melts at 310°C; boils at 707°C; insoluble in water; soluble in liquid ammonia and alkalies.출처
Arsenic sesquisulfide occurs in nature as the mineral orpiment. It is used as a pigment; in the manufacture of infrared-transmitting glass; in semiconductors and photoconductors; in pyrotechnics; in linoleum and oil cloth; for the removal of hairs from hides; and as a reducing agent.용도
manufacture of glass, particularly infrared-transmitting glass; manufacture of oil cloth, linoleum; in electrical semiconductors, photoconductors; as pigment; for depilating hides; in pyrotechnics.정의
Arsenic(III) sulfide (Arsenic tri-sulfide glass) is a glassy material. It is important as the window of a cell for a sample containing water.제조 방법
Arsenic sesquioxide may be prepared by heating arsenic trioxide with hydrogen sulfide:As2O3 + 3 H2S → As2S3 + 3 H2O
Alternatively, it may be precipitated out from a solution of arsenous acid or arsenic trioxide in dilute hydrochloric acid by passing hydrogen sulfide into the solution:
2H3AsO3 + 3H2S → As2S3 + 6H2O.
일반 설명
A yellow or red crystalline solid or powder. Combustible. Insoluble in water. Toxic by inhalation (dust) and ingestion.공기와 물의 반응
Insoluble in water.반응 프로필
ARSENIC (III) SULFIDE is dissolved by alkalis and by hydrochloric acid (slowly). Decomposed by nitric acid. May react with oxidizing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. The reactions may be violent. ARSENIC (III) SULFIDE reacts with concentrated solutions of chloric acid with incandescence [Mellor Supp. II Part I:584 1956]. Generates toxic gaseous hydrogen sulfide on contact with acids.건강위험
(Acute and sub-acute poisoning are not common.) Repeated inhalation causes irritation of nose, laryngitis, mild bronchitis. Ingestion causes weakness, loss of appetite, gastrointestinal disturbances, peripheral neuritis, occasional hepatitis. Contact with eyes causes irritation. Irritates skin, especially where moist; if not treated, may cause ulceration.화재위험
Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.공업 용도
As2S3 (orpiment) and As2S2 (realgar) have been used as early as 2000 BC as drugs, for example, to cure cancerous tumours, ulcers and other diseases of the time. Later, Galen (130 200 AD) recommended the application of a paste of arsenic sulfide against ulcers.Safety Profile
Confirmed human carcinogen with experimental tumorigenic data. A poison. Reacts violently with H202, (m03 + S). When heated to decomposition or on contact with acid or acid fumes it emits highly toxic fumes of S02, H2S, and As. Reacts with water or steam to emit toxic and flammable vapors.잠재적 노출
Arsenic trisulfide is used in the manufacture of glass, oil cloth, linoleum, electrical semiconductors, fireworks and used as a pigment.운송 방법
UN1557 Arsenic compounds, solid, n.o.s. inorganic, including arsenates, n.o.s.; arsenites, n.o.s.; arsenic sulfides, n.o.s.; and organic compounds of arsenic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials.비 호환성
A strong reducing agent; avoid contact with oxidizers (such as perchlorates, peroxides, permanganates, chlorates, and nitrates) and potassium nitrate mixed with sulfur, since violent reactions occur. Water contact forms hydrogen sulfide. Incompatible with acids, halogens. Contact with acids or acid mists releases deadly arsine gas.폐기물 처리
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.비소 황화합물 준비 용품 및 원자재
원자재
준비 용품
비소 황화합물 공급 업체
글로벌( 110)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 |
info@antaichem.com | CHINA | 9641 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 |
sales@hzclap.com | CHINA | 6313 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 |
1026@dideu.com | China | 29220 | 58 |
| Henan Alfa Chemical Co., Ltd | +8618339805032 |
alfa4@alfachem.cn | China | 12755 | 58 |
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 |
sales01@sdlookchemical.com | China | 2739 | 58 |