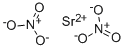질산 스트론튬
|
|
질산 스트론튬 속성
- 녹는점
- 570 °C (lit.)
- 끓는 점
- 645 °C
- 밀도
- 2.99
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- 660g/L
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- Specific Gravity
- 2.99
- 색상
- 하얀색
- 수소이온지수(pH)
- 5-7 (50g/l, H2O, 20℃)
- 냄새
- 냄새 없는
- pH 범위
- 5.0 - 7.0
- 수용성
- 660g/L(20℃)
- 감도
- Hygroscopic
- Merck
- 14,8845
- 안정성
- 강산화제 - 가연성 물질과 접촉하면 화재가 발생할 수 있습니다. 강력한 환원제, 가연성 물질과 혼합할 수 없습니다.
- InChIKey
- DHEQXMRUPNDRPG-UHFFFAOYSA-N
- CAS 데이터베이스
- 10042-76-9(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | O,Xi,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 8-36/37/38-22 | ||
| 안전지침서 | 17-26-36/37/39-36 | ||
| 유엔번호(UN No.) | UN 1507 5.1/PG 3 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | WK9800000 | ||
| TSCA | Yes | ||
| 위험 등급 | 5.1 | ||
| 포장분류 | III | ||
| HS 번호 | 28342980 | ||
| 유해 물질 데이터 | 10042-76-9(Hazardous Substances Data) | ||
| 독성 | LD50 i.p. in rats: 540 mg/kg, Cochran et al., Arch. Ind. Hyg. 1, 637 (1950) | ||
| 기존화학 물질 | KE-32235 |
질산 스트론튬 C화학적 특성, 용도, 생산
개요
Strontium nitrate has the molecular formula of Sr(NO3)2 and the molecular weight of 211.6327 g/mol. Strontium nitrate is prepared by treating strontium carbonate with nitric acid. The solution is evaporated and crystallized:SrCO3 +HNO3 ? Sr(NO3)2 + CO2 +H2O
Crystallization yields the tetrahydrate, Sr(NO3)2· 4H2O, which, on heating, melts at 31.3 °C in its own waters of hydration and then decomposes >100 °C to form the anhydrous nitrate. The anhydrate melts at 570 °C (It begins to decompose as low as 545 °C), is soluble in water (70.3 gm/100 ml at 20 °C) and is very slightly soluble in ethanol. These colorless crystals, or white powder, have a density of 2.986 g/cm3 and its CAS number is 10042-76-9. It is very soluble in water, as shown in Table 4.11 on the following page. Strontium nitrate apparently forms only the tetrahydrate when a solution is evaporated below 80°C. Otherwise it decomposes at higher solution temperatures to Sr(OH)2 and nitrogen oxides. The nitric acid concentration makes little difference in the hydrate produced, in contrast to other alkaline earths like Ca and Mg.
화학적 성질
Strontium nitrate is a white crystalline solid물리적 성질
Colorless cubic crystals or white powder or granules; density 2.986 g/cm3; melts at 570°C; very soluble in water, 80 g/100 mL at 18°C; very slightly soluble in ethanol.The tetrahydrate constitutes colorless monoclinic crystals; density 2.20 g/cm3; loses all water of crystallization at 100°C; converts to strontium oxide, SrO at 1,100°C; very soluble in water, 60.4g/100 mL at 0°C, 206 g/100 mL at 100°C; soluble in liquid ammonia; very slightly soluble in ethanol and acetone.
용도
Strontium nitrate is used in pyrotechnics, for producing marine and railroad signals, and in matches.제조 방법
Strontium nitrate is prepared by treating strontium carbonate with nitric acid. The solution is evaporated and crystallized:SrCO3 + HNO3 → Sr(NO3)2 + CO2 + H2O
Crystallization yields the tetrahydrate, Sr(NO3)2•4H2O, which on heating dehydrates to form the anhydrous nitrate.
일반 설명
A white crystalline solid. Noncombustible but accelerates burning of combustible materials. May explode if large quantities are involved in a fire or the combustible material is finely divided. May explode under prolonged exposure to heat or fire. Toxic oxides of nitrogen are produced in fires. Used in pyrotechnics, in medicine, and to make other chemicals.공기와 물의 반응
Soluble in water.반응 프로필
Mixtures of metal/nonmetal nitrates with alkyl esters may explode, owing to the formation of alkyl nitrates; mixtures a nitrate with phosphorus, tin (II) chloride, or other reducing agents may react explosively [Bretherick 1979 p. 108-109]. Special Hazards of Combustion Products: Yields toxic gaseous oxides of nitrogen when involved in fire.건강위험
Dust is irritating to skin, eyes, and respiratory system.Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. A powerful oxidizer. When heated to decomposition it emits toxic fumes of NOx.See also NITRATES and STRONTIUM COMPOUNDS.잠재적 노출
Strontium nitrate is used in matches, pyrotechnics, marine signals; and railroad flares.운송 방법
UN1507 Strontium nitrate, Hazard Class: 5.1; Labels: 5.1-Oxidizer.Purification Methods
Crystallise it from hot water (0.5mL/g) by cooling to 0o.비 호환성
A strong oxidizer. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Violent reaction with reducing agents; combustibles, organics, or other readily oxidizable materials. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.질산 스트론튬 준비 용품 및 원자재
원자재
준비 용품
질산 스트론튬 관련 검색:
스트론튬 질산 코발트 질산 바륨 질산 수소화 스트론튬 질산 스트론튬 질산 칼슘 질산나트륨 질산칼륨 질산암모늄
Miconazole Nitrate
Strontium ranelate
NITRATE
Econazole nitrate
STRONTIUM, STANDARD SOLUTION 1000 MG/L SR FOR AA (STRONTIUM NITRATE IN NITRIC ACID 0,5 MOL/L)
STRONTIUM NITRATE (SR(NO3)2) 99.995% 2G
STRONTIUM NITRATE ANHYDROUS
STRONTIUM NITRATE (SR(NO3)2) 99.995% 10G