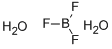Boron trifluoride dihydrate C화학적 특성, 용도, 생산
화학적 성질
colourless liquid
일반 설명
Boron trifluoride dihydrate is a fuming liquid. Boron trifluoride dihydrate may be corrosive to skin, eyes and mucous membranes. Boron trifluoride dihydrate may be toxic by inhalation. Boron trifluoride dihydrate is used as a catalyst in chemical reactions.
공기와 물의 반응
In moist air forms dense white fumes pungent, corrosive to skin, avoid inhalation [Merck 11th ed. 1989].
반응 프로필
BORON TRIFLUORIDE is a colorless, strongly irritating, liquid. Upon contact with water, steam or when heated to decomposition, Boron trifluoride dihydrate will produce toxic fluoride fumes. Incompatible with alkyl nitrates, calcium oxide. Reaction with alkali metals or alkaline earth metals (except magnesium) will cause incandescence [Bretherick, 5th ed., 1995, p. 65].
건강위험
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
화재위험
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
잠재적 노출
Boron trifluoride is a highly reactive
chemical used primarily as a catalyst in chemical synthesis.
It is stored and transported as a gas, but can be reacted
with a variety of materials to form both liquid and solid
compounds. The magnesium industry utilizes the fireretardant
and antioxidant properties of boron trifluoride
in casing and heat treating. Nuclear applications of boron
trifluoride include neutron detector instruments; boron-10
enrichment and the production of neutroabsorbing salts for
molten-salt breeder reactors.
운송 방법
UN1008 Boron trifluoride, Hazard class: 2.3;
Labels: 2.3—Poisonous gas, 8—Corrosive material,
Inhalation Hazard Zone B. Cylinders must be transported
in a secure upright position, in a well-ventilated truck.
Protect cylinder and labels from physical damage. The
owner of the compressed gas cylinder is the only entity
allowed by federal law (49CFR) to transport and refill
them. It is a violation of transportation regulations
to refill compressed gas cylinders without the express
written permission of the owner.
비 호환성
Boron trifluoride reacts with polymerized
unsaturated compounds. Decomposes on contact with
water, moist air, and other forms of moisture, forming toxic
and corrosive hydrogen fluoride, fluoroboric acid, and boric
acid. Reacts violently with alkali and alkaline earth metals
(except magnesium); metals, such as sodium, potassium,
and calcium oxide, and with alkyl nitrates. Attacks many
metals in presence of water.
폐기물 처리
Return refillable compressed
gas cylinders to supplier. The owner of the compressed gas
cylinder is the only entity allowed by federal law (49CFR)
to transport and refill them. Chemical reaction with water
to form boric acid, and fluoroboric acid. The fluoroboric
acid is reacted with limestone, forming boric acid and calcium
fluoride. The boric acid may be discharged into a sanitary
sewer system while the calcium fluoride may be
recovered or landfilled. Protect cylinder and labels from
physical damage.
Boron trifluoride dihydrate 준비 용품 및 원자재
원자재
준비 용품