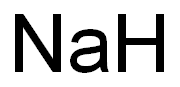나트륨
|
|
나트륨 속성
- 녹는점
- 97.8 °C (lit.)
- 끓는 점
- 883 °C (lit.)
- 밀도
- 1.04 g/mL at 20 °C
- 증기압
- 1 mm Hg ( 440 °C)
- 인화점
- 128 °F
- 저장 조건
- water-free area
- 용해도
- H2O: 용해성
- 물리적 상태
- 플레이크 크리스탈(대형)
- Specific Gravity
- 0.97
- 색상
- 흰색에서 황백색까지
- 비저항
- 4.69 μΩ-cm, 20°C
- 수용성
- 물과 반응
- 감도
- Air & Moisture Sensitive
- Merck
- 14,8570
- 노출 한도
- ACGIH: TWA 2 ppm; STEL 4 ppm
OSHA: TWA 2 ppm(5 mg/m3)
NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3)
- 안정성
- 물과 격렬하게 반응하여 수소를 방출하고 발화할 수도 있음. 가연성 고체. 물, 강한 산화제와 호환되지 않습니다. 산화제 근처에 보관하지 마십시오. 기름이나 건조한 불활성 가스 아래에 보관하십시오. 공기에 민감합니다.
- InChIKey
- MPMYQQHEHYDOCL-UHFFFAOYSA-N
- CAS 데이터베이스
- 7440-23-5(CAS DataBase Reference)
- NIST
- Sodium(7440-23-5)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,F,T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 34-14/15-45-65 | ||
| 안전지침서 | 26-8-6A-45-43D-43-36/37/39-22-7/8-62-5 | ||
| 유엔번호(UN No.) | UN 3264 8/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | VY0686000 | ||
| 자연 발화 온도 | >115 °C in air | ||
| TSCA | Yes | ||
| HS 번호 | 2805 11 00 | ||
| 위험 등급 | 4.3 | ||
| 포장분류 | I | ||
| 유해 물질 데이터 | 7440-23-5(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-31338 | ||
| 유해화학물질 필터링 | 97-1-7 | ||
| 사고대비 물질 필터링 | 41 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 나트륨 및 이를 25% 이상 함유한 혼합물 |
나트륨 C화학적 특성, 용도, 생산
개요
In its ionic form, sodium is one of the most important biological nutrients and is found nearly everywhere on Earth. Although it was isolated as a free metal in 1807 by Sir Humphry Davy and makes up 2.83% of Earth’s lithosphere, it is not found in its metallic form in nature. Pure sodium is extremely reactive, particularly with water to form explosive hydrogen gas and lye (NaOH); it can also react with water vapor in air or biological tissues.Mined and refined salts from terrestrial and aquatic sources contain sodium in the form of sodium chloride, sodium iodide, and other compounds. Natron, a naturally occurring mixture of sodium compounds, has been used since the time of the ancient Egyptians, and sodium compounds are essential to numerous industries, including those involving glass, paper, and soap production. Since it does not occur in its metallic form in nature, pure sodium metal must be produced industrially, which is accomplished via electrolysis of molten sodium chloride.
화학적 성질
Sodium is a soft silvery white metallic element. Pyrophoric solid or molten liquid. Odorless, oxidizing rapidly in air; waxlike at room temperature, brittle at low temperatures. Store in airtight containers or in naphtha or similar liquid that does not contain water or free oxygen. Decomposes water on contact, with evolution of hydrogen to form sodium hydroxide; insoluble in benzene, kerosene, and naphtha. Has excellent elec- trical conductivity and high heat-absorbing capacity.물리적 성질
Sodium is a soft, wax-like silver metal that oxidizes in air. Its density is 0.9674 g/cm3, andtherefore it floats on water as it reacts with the water releasing hydrogen. It has a rather lowmelting point (97.6°C) and a boiling point of 883°C. Sodium is an excellent conductor ofheat and electricity. It looks much like aluminum but is much softer and can be cut with aknife like butter. Its oxidation state is +1.Isotopes
Sodium has 14 isotopes. The only stable isotope of sodium has an averageatomic weight of 23 (23Na) and makes up about 100% of all the isotopes of the element sodium found on Earth. All the other 13 isotopes (from 19Na to 31Na) are radioactive with relatively short half-lives and thus are unstable.Origin of Name
The Latin name for the symbol for “sodium” (Na) is natrium, and the name “sodium” in Latin is sodanum, which was known as an ancient headache remedy and was called “soda” in English.출처
Sodium is the sixth most abundant of the Earth’s elements. Since it is a highly electropositive metal and so reactive with nonmetals, it is not found in its pure elemental form on Earth.Rather, it is found in numerous compounds in relatively abundant quantities. About 2.83%of the Earth’s crust consists of sodium in compounds.Sodium is produced by an electrolytic process, similar to the other alkali earth metals. (Seefigure 4.1). The difference is the electrolyte, which is molten sodium chloride (NaCl, common table salt). A high temperature is required to melt the salt, allowing the sodium cationsto collect at the cathode as liquid metallic sodium, while the chlorine anions are liberated aschlorine gas at the anode: 2NaCl (salt) + electrolysis → Cl2↑ (gas) + 2Na (sodium metal). Thecommercial electrolytic process is referred to as a Downs cell, and at temperatures over 800°C,the liquid sodium metal is drained off as it is produced at the cathode. After chlorine, sodiumis the most abundant element found in solution in seawater.Characteristics
On the periodic table sodium is located between lithium and potassium. A fresh cut intosodium looks silvery but turns gray as sodium oxidizes rapidly in air, forming sodium oxideon its surface.Sodium is extremely reactive. It reacts explosively in water as it releases hydrogen fromthe water with enough heat to ignite the hydrogen. The resulting compound of this reactionis sodium hydroxide (2Na + 2H2O → 2NaOH + H2↑). Due to its extremely electropositivereactivity, there are few uses for the pure metallic form of sodium. Because of its reactivity,hundreds of sodium compounds are found on the Earth’s surface.Guide to the Elements | 51An unusual characteristic of several alkali metals is that a mixture of two or more has alower melting point than the melting point of the separate metals. This is referred to as aeutectic system of metallic alloys. For instance, sodium has a melting point of 97.6°C, andpotassium’s melting point is 63.25°C, but when the two are mixed, the eutectic melting point(turning into a liquid phase) of the combined Na-K system is below zero degrees Celsius(–10°C). If cesium metal (melting point of 38.89°C) is added to the Na and K mixture, themelting point of this eutectic alloy (Na-K-Cs) is the lowest of any eutectic alloy at –78°C.용도
manufacture of sodium Compounds, such as the cyanide, azide, peroxide, etc.; manufacture of tetraethyllead; manufacture of refractory metals; in org syntheses; for photoelectric cells; in sodium lamps; as catalyst for many polymerization reactions. Alloyed with potassium in heat transfer media.생산 방법
Sodium is an essential element needed for all organic life. Sodium is produced commercially through the electrolysis of liquid sodium chloride mixed with calcium chloride in a Downs Cell. Very pure sodium can be isolated by the thermal decomposition of sodium azide (NaN3). Sodium, in its metallic form, can be used to refine some reactive metals, such as zirconium and potassium, from their compounds and is very important in making esters.정의
sodium: Symbol Na. A soft silveryreactive element belonging to group1 (formerly IA) of the periodic table(see alkali metals); a.n. 11; r.a.m.22.9898; r.d. 0.97; m.p. 97.8°C; b.p.882–889°C. Sodium occurs as thechloride in sea water and in the mineralhalite. It is extracted by electrolysisin a Downs cell. The metal isused as a reducing agent in certainreactions and liquid sodium is also acoolant in nuclear reactors. Chemically,it is highly reactive, oxidizingin air and reacting violently withwater (it is kept under oil). It dissolvesin liquid ammonia to formblue solutions containing solvatedelectrons. Sodium is a major essentialelement required by living organisms.The element was first isolatedby Humphry Davy in 1807.일반 설명
Sodium,Na, melts at 97.8°C and boils at 892°C. It is silver-white in color, is soft and malleable, and oxidizes in air. When exposed to air, a silvery soft metal that becomes grayish white upon. It occurs naturally only in the forms of its salts. Shipped as a solid or molten liquid. Burns violently with explosions that may spatter the material. Sodium is used as a chemical intermediate. and in pharmaceuticals, petroleum refining and metallurgy, electric power cable, Sodium lamps, other chemicals.공기와 물의 반응
May ignite spontaneously in air. Reacts violently with water to give Sodium hydroxide and hydrogen, which ignites spontaneously [Merck, 11th ed. 1989)]. The ignition temperature of Sodium in air depends on the area of surface exposed: vapor ignites at room temperature; droplets at about 250°F; an agitated pool at 400°F. In the absence of moisture and hydrogen, the reaction is insignificant [Mellor 2 Supp. 2:440 1961].위험도
Sodium as the elemental metal is very dangerous because of its extreme electropositivenature, particularly when it comes in contact with moist air, water, snow, or ice or otheroxidizing agents. It readily gives up electrons to electronegative atoms (nonmetals). In thesereactions, it releases hydrogen gas with enough heat to explosively ignite the hydrogen.Numerous sodium compounds are hazardous as carcinogens (cancer-causing) and astoxins (poisons) in plants and animals. On the other hand, we benefit greatly from the manycompounds containing the element sodium. We could not live without it.
건강위험
Sodium reacts with the moisture on skin and other tissues to form highly corrosive sodium hydroxide. Contact of metallic sodium with the skin, eyes, or mucous membranes causes severe burns; thermal burns may also occur due to ignition of the metal and liberated hydrogen.화재위험
Sodium spontaneously ignites when heated above 115 °C in air that has even modest moisture content, and any sodium vapor generated is even more flammable. Sodium reacts violently on contact with water and often ignites or explodes the hydrogen formed. Sodium fires must be extinguished with a class D dry chemical extinguisher or by the use of sand, ground limestone, dry clay or graphite, or ''Met- L-X ? " type solids. Water or CO 2 extinguishers must never be used on sodium fires.인화성 및 폭발성
Sodium spontaneously ignites when heated above 115 °C in air that has even modest moisture content, and any sodium vapor generated is even more flammable. Sodium reacts violently on contact with water and often ignites or explodes the hydrogen formed. Sodium fires must be extinguished with a class D dry chemical extinguisher or by the use of sand, ground limestone, dry clay or graphite, or "Met- L-X ?" type solids. Water or CO2 extinguishers must never be used on sodium fires.잠재적 노출
A potential danger to those involved in tetra-alkyl lead manufacture using lead-sodium alloy as a reactant; those using sodium as a liquid metal coolant, as a catalyst, or in the manufacture of sodium hydride, borohydride, or peroxide.환경귀착
Elemental sodium that is released into the environment reacts almost immediately with water to form sodium hydroxide and hydrogen gas. Even small quantities of metallic sodium can be explosive when brought into contact with sources of water; the formation sodium hydroxide raises the local pH and is extremely caustic. Sodium cations formed from this reaction are rapidly absorbed into the surrounding environment to form a large variety of salts.저장
Safety glasses, impermeable gloves, and a fire-retardant laboratory coat should be worn at all times when working with sodium, and the metal should be handled under the surface of an inert liquid such as mineral oil, xylene, or toluene. Sodium should be used only in areas free of ignition sources and should be stored under mineral oil in tightly sealed metal containers under an inert gas such as argon.운송 방법
UN1428 Sodium, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material. Note: Finely divided sodium is pyrophoric.Purification Methods
The metal is placed on a coarse grade of sintered-glass filter, melted under vacuum and forced through the filter using argon. The Pyrex apparatus is then re-evacuated and sealed off below the filter, so that the sodium could be distilled at 460o through a side arm and condenser into a receiver bulb which is then sealed off [Gunn & Green J Am Chem Soc 80 4782 1958]. EXPLODES and IGNITES in water.비 호환성
A strong reducing agent. A dangerous fire hazard when exposed to heat and moisture. Violent reaction with water, forming NaOH. Violent reaction with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. halogenated hydrocarbons; phosphorus and phosphorus compounds; sulfur and sulfur compounds; and many other chemicals.폐기물 처리
Incineration with absorption of oxide fumes.나트륨 준비 용품 및 원자재
원자재
준비 용품
SODIUM PHENOLATE TRIHYDRATE
Ethyl alpha-Formyl Benzeneacetic Acid Ester
10-(METHYLSULFONYL)CAPRAMIDE
3,3-Diphenylpropanol
ETHYL PICOLINOYLACETATE
6-AMINO-2-METHYLTHIO-3-METHYLURACIL
2-Amino-4-methoxypyridine
4-Methylbenzene-1-carboximidamide hydrochloride
2,4-DIMETHYLQUINOLINE
아밀 메틸 카비놀
2-메톡시퓨랸
4- 아미노 -2- 메틸 피리 미딘 -5- 카보
4-AMINO-2-MERCAPTOPYRIMIDINE-5-CARBONITRILE
2-Amino-6-hydroxypyrimidin-4(3H)-one ,97%
Tiotropium bromide
ETHYL 3-AMINOTHIOPHENE-2-CARBOXYLATE
4-METHOXY-BENZAMIDINE
2-AMINO-4-METHOXYPYRIMIDINE
4,4,4-Trifluoro-1-phenyl-1,3-butanedione
4-AMINO-2-(TRIFLUOROMETHYL)PYRIMIDINE-5-CARBOXYLIC ACID
4,6-DIAMINO-2-MERCAPTOPYRIMIDINE
4-AMINO-2-METHYL-PYRIMIDINE-5-CARBOXYLIC ACID
bis(2-ethylhexyl) phenyl phosphite
트리페닐틴 클로라이드
5-BROMO-2-CHLORO-4-METHOXYPYRIMIDINE
2-amino-6-chloropyrimidin-4(3H)-one
이소프로필글리시딜에테르
4-Chlorobenzene-1-carboximidamide hydrochloride
4-METHYL-BENZAMIDINE
4-CHLOROBENZAMIDINE HYDROIODIDE
과산화나트륨
4-AMINOPYRIMIDINE-5-CARBONITRILE
6-AMINO-1-METHYL-5-NITROSOURACIL
3-OCTANOL
2,2'-DIPYRIDYLAMINE
4-METHOXYBENZAMIDINE, HYDROCHLORIDE
2,4-DIOXO-4-THIOPHEN-2-YL-BUTYRIC ACID METHYL ESTER
2-ETHOXYPYRIMIDIN-4-YLAMINE
4-Methyl-2-thiophenecarboxylic acid
2-Methyl-1-butanethiol