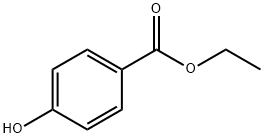
에틸파라벤
|
|
에틸파라벤 속성
- 녹는점
- 114-117 °C (lit.)
- 끓는 점
- 297-298 °C (lit.)
- 밀도
- 1.1708 (rough estimate)
- 증기압
- 0.00012 hPa (25 °C)
- 굴절률
- 1.5286 (estimate)
- 인화점
- 297-298°C
- 저장 조건
- 2-8°C
- 용해도
- 물에는 매우 약간 용해되며, 에탄올(96%) 및 메탄올에는 잘 용해됩니다.
- 물리적 상태
- 결정성 분말
- 산도 계수 (pKa)
- 8.31±0.13(Predicted)
- 색상
- 하얀색
- 수소이온지수(pH)
- 4.5-5.5 (H2O, 20°C) (saturated solution)
- 냄새
- 100.00?%. 약한 페놀성
- 수용성
- 물에 약간 용해됩니다.
- Merck
- 14,3837
- BRN
- 1101972
- 안정성
- 안정적인. 타기 쉬운. 강한 산화제, 강한 염기와 호환되지 않습니다.
- LogP
- 2.470
- CAS 데이터베이스
- 120-47-8(CAS DataBase Reference)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 26-36-24/25 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | DH2190000 | ||
| 자연 발화 온도 | 495 °C | ||
| TSCA | Yes | ||
| HS 번호 | 29182930 | ||
| 유해 물질 데이터 | 120-47-8(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: > 2000 mg/kg | ||
| 기존화학 물질 | KE-13823 |
에틸파라벤 C화학적 특성, 용도, 생산
순도시험
(1) 융점 : 이 품목의 융점은 115~118℃이어야 한다.
(2) 유리산 : 이 품목 0.75g에 물 15mL를 가하여 비등수욕 중에서 1분간 가열하고 식힌 다음 여과한 액은 산성 또는 중성이다. 여액 10mL를 취하여 0.1N 수산화나트륨용액 0.2mL 및 메틸레드시액 2방울을 가할 때, 그 액은 황색을 나타낸다.
(3) 황산염 : 이 품목 1g에 열탕 100mL를 가하여 잘 흔들어 섞으면서 5분간 가열하고 식힌 다음 물을 가하여 100mL로 하여 여과한 다음 여액 40mL에 묽은염산 1mL를 가하고 이를 시험용액으로 하여 황산염시험법에 따라 시험할 때, 그 양은 0.01N 황산 0.2mL에 대응하는 양 이하이어야 한다.
(4) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(5) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 2.0ppm 이하이어야 한다.
(6) 수은 : 이 품목을 수은시험법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(7) 파라옥시안식향산 및 살리실산 : 이 품목 0.5g을 정밀히 달아 에테르 30mL에 녹이고 탄산수소나트륨용액(1→100) 20mL를 가한 다음 진탕한 후 물층을 취한다. 물층을 에테르 20mL씩으로 2회 세정한 후, 묽은황산 5mL 및 에테르 30mL를 가해 주고 진탕한 다음 에테르층을 취하고 물 10mL로 세정한다. 에테르층을 여과하고 용기 및 필터를 소량의 에테르로 씻어 합한 다음 수욕상에서 증발건고한 후 황산데시케이터에서 항량이 될 때까지 건조하여 잔류물의 양을 구할 때, 그 양은 5mg을 초과해서는 아니되며 잔류물을 물 25mL에 녹이고 70℃로 가열한 다음 여과한 후 묽은염화제이철시액을 가할 때, 자~적자색을 나타내어서는 아니 된다.
확인시험
(1) 이 품목 0.5g에 수산화나트륨시액 10mL를 가하고 30분간 끓인 다음 증발 농축하여 약 5mL로 하고 식힌 다음 묽은황산으로 산성으로 하여 생성된 침전을 여취하여 물로 잘 씻고 105℃에서 1시간 건조할 때, 그 융점은 213~217℃이다.
(2) 이 품목 0.05g에 초산 2방울 및 황산 5방울을 가하여 5분간 가온할 때, 액은 초산에틸의 냄새를 발생한다.
정량법
이 품목 약 2g을 정밀히 달아 이에 1N 수산화나트륨용액 40mL를 가하여 30분간 끓이고 식힌 다음 과잉의 알칼리를 1N 황산으로 적정한다(지시약 : 브로모티몰블루시액 5방울). 종말점의 색은 pH 6.5의 완충액에 같은 지시약을 가한 때의 색으로 한다. 따로 같은 방법으로 공시험을 한다.
1N 수산화나트륨용액 1mL = 166.2mg C9H10O3
강열잔류물
이 품목 5g을 달아 강열잔류물시험법에 따라 시험할 때, 그 양은 0.05% 이하이어야 한다.
화학적 성질
Ethylparaben occurs as a white, odorless or almost odorless, crystalline powder.용도
Ethylparaben is mainly used as antiseptics in cosmetics, food and medicine. It is also can be used as feed preservatives and antiseptic for bacteria.Ethylparaben is a preservative that is used in the formulation of cosmetics and personal care products in order to extend the shelf-life by preventing microbial contamination. In most formulations, parabens are used at very low levels ranging from 0.01 to 0.3%.
Ethylparaben, a novel sorbent for solid-phase extraction, was used to study its retention property. It has high extraction efficiency towards the compounds tested owing to the electrostatic interaction, hydrophobic interaction and hydrogen bonding.
생산 방법
Ethylparaben is prepared by the esterification of p-hydroxybenzoic acid with ethanol (95%).Pharmaceutical Applications
Ethylparaben is widely used as an antimicrobial preservative in cosmetics,food products, and pharmaceutical formulations.It may be used either alone or in combination with other paraben esters or with other antimicrobial agents. In cosmetics it is one of the most frequently used preservatives.
The parabens are effective over a wide pH range and have a broad spectrum of antimicrobial activity, although they are most effective against yeasts and molds.
Owing to the poor solubility of the parabens, paraben salts, particularly the sodium salt, are frequently used. However, this may cause the pH of poorly buffered formulations to become more alkaline.
Safety
Ethylparaben and other parabens are widely used as antimicrobial preservatives in cosmetics, food products, and oral and topical pharmaceutical formulations.Systemically, no adverse reactions to parabens have been reported, although they have been associated with hypersensitivity reactions. Parabens, in vivo, have also been reported to exhibit estrogenic responses in fish.(10) The WHO has set an estimated total acceptable daily intake for methyl-, ethyl-, and propylparabens at up to 10 mg/kg body-weight.
LD50 (mouse, IP): 0.52 g/kg
LD50 (mouse, oral): 3.0 g/kg
저장
Aqueous ethylparaben solutions at pH 3–6 can be sterilized by autoclaving, without decomposition. At pH 3–6, aqueous solutions are stable (less than 10% decomposition) for up to about 4 years at room temperature, while solutions at pH 8 or above are subject to rapid hydrolysis (10% or more after about 60 days at room temperature).Ethylparaben should be stored in a well-closed container in a cool, dry place.
비 호환성
The antimicrobial properties of ethylparaben are considerably reduced in the presence of nonionic surfactants as a result of micellization. Absorption of ethylparaben by plastics has not been reported, although it appears probable given the behavior of other parabens. Ethylparaben is coabsorbed on silica in the presence of ethoxylated phenols. Yellow iron oxide, ultramarine blue, and aluminum silicate extensively absorb ethylparaben in simple aqueous systems, thus reducing preservative efficacy.Ethylparaben is discolored in the presence of iron and is subject to hydrolysis by weak alkalis and strong acids.
Regulatory Status
Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral, otic, and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.에틸파라벤 준비 용품 및 원자재
원자재
준비 용품
에틸파라벤 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12453 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 |
bj1@bj-chem.com | China | 298 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| Hubei XinRunde Chemical Co., Ltd. | +8615102730682 |
bruce@xrdchem.cn | CHINA | 566 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Tianjin Zhongxin Chemtech Co., Ltd. | +86-022-66880623 +8618622897568 |
sales@tjzxchem.com | China | 559 | 58 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |