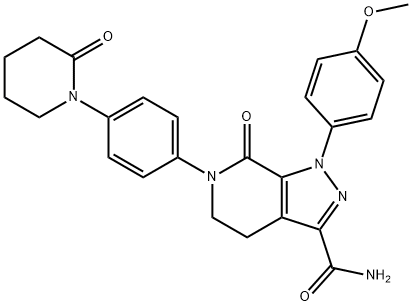
Apixaban
|
|
Apixaban 속성
- 녹는점
- 235-238°C
- 끓는 점
- 770.5±60.0 °C(Predicted)
- 밀도
- 1.42
- 저장 조건
- Refrigerator
- 용해도
- DMSO(약간 용해됨, 가열), 메탄올(약간 용해됨)
- 산도 계수 (pKa)
- 15.01±0.20(Predicted)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 흰색에서 황백색까지
- InChIKey
- QNZCBYKSOIHPEH-UHFFFAOYSA-N
- SMILES
- C1(=O)N(C2=CC=C(N3CCCCC3=O)C=C2)CCC2C(C(N)=O)=NN(C3=CC=C(OC)C=C3)C1=2
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 유해 물질 데이터 | 503612-47-3(Hazardous Substances Data) |
|---|
| 그림문자(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Warning | ||||||||||||||
| 유해·위험 문구: |
|
||||||||||||||
| 예방조치문구: |
|
Apixaban C화학적 특성, 용도, 생산
개요
Eliquis (apixaban), a direct inhibitor of factor Xa (FXa), was approved by the European Commission on May 18, 2011 for prevention of venous thromboembolic events (VTE) in adult patients who have undergone elective hip or knee replacement surgery. The discovery of apixaban was the culmination of a succession of novel and innovative medicinal chemistry discoveries starting with the identification of nonpeptide leads, rational drug design using computer-aided and X-ray crystallographic information, and the building of drug-like properties through the systematic replacement of basic groups with neutral moieties. Apixaban arose from modifications to razaxaban by constraining a pyrazole amide to form a bicyclic pyrazolo-pyridinone scaffold. Optimization of the P1 group resulted in the identification of the nonbasic methoxy phenyl group, while a P4 piperidinone improved the balance of potency and pharmacokinetics with low Vdss. The synthesis of apixaban begins with the generation of a hydrazone of 4-methoxyaniline which is then used in a 3+2 cycloaddition with a dihydropiperidinone to form a bicyclic pyrazolo-pyridinone scaffold. The distal piperidinone group is installed using an Ullmann coupling reaction followed by aminolysis of an ethyl ester on the pyrazole ring to complete the synthesis of apixaban.용도
Apixaban is a highly selective, reversible inhibitor of Factor Xa with Ki of 0.08 nM and 0.17 nM in human and rabbit, respectively.정의
ChEBI: A pyrazolopyridine that is 7-oxo-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide substituted at position 1 by a 4-methoxyphenyl group and at position 6 by a 4-(2-oxopiperidin-1-yl)phenyl group. It is used for the prevention and treatment of thromboembolic diseases.Pharmacokinetics
The maximum plasma concentration (Cmax) of apixaban occurs 3–4 h after oral administration. The absorption of apixaban appears to occur primarily in the small intestine and decreases progressively throughout the gastrointestinal tract. Compared with oral administration, the bioavailability of 2.5 mg of apixaban solution was approximately 60% and 84% lower when released in the distal small bowel and ascending colon, respectively. For oral doses up to 10 mg, the absolute bioavailability of apixaban is~50%, resulting from the incomplete absorption and first-pass metabolism in the gut and liver[1].Clinical Use
Apixaban is an oral anticoagulant with highly selective inhibition of factor Xa. It was approved by the European Medicines Agency (EMA) for the treatment of venous thromboembolic events and first marketed in Germany under the brand name Eliquis in June 2011. Apixaban was co-developed by Bristol-Myers Squibb and Pfizer and represents the first approved drug for this indication since warfarin over 50 years ago.부작용
Possible side effects of Apixaban are: bleeding gums, nosebleeds, heavy vaginal bleeding , red, pink, or brown urine; red or black, tarry stools; coughing or spitting up blood or a substance that looks like coffee grounds; swelling or joint pain, headache, rash, chest pain or tightness in the chest, swelling of the face or tongue, trouble breathing, wheezing. Feeling dizzy or fainting.Apixaban prevents your blood from clotting properly, so if you get a cut or injury, it may take longer than usual for the bleeding to stop. This medication may also cause you to bruise or bleed more easily.참고 문헌
https://en.wikipedia.org/wiki/Apixabanhttps://www.drugbank.ca/drugs/DB06605
[1] Wonkyung Byon. “Apixaban: A Clinical Pharmacokinetic and Pharmacodynamic Review.” Clinical Pharmacokinetics 58 10 (2019): 1265–1279.
Apixaban 준비 용품 및 원자재
원자재
준비 용품
Apixaban 공급 업체
글로벌( 783)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Jinan Million Pharmaceutical Co., Ltd | +86-531-68659554 +8613031714605 |
info@millionpharm.com | China | 154 | 58 |
| Jinan Jianfeng Chemical Co., Ltd | 0531-88110457; +8615562555968 |
info@pharmachemm.com | China | 222 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 |
info@afinechem.com | CHINA | 15377 | 58 |
| APOLLO HEALTHCARE RESOURCES | +6596580999 |
sales@apollo-healthcare.com.sg | Singapore | 400 | 58 |
| Changzhou Rokechem Technology Co., Ltd. | 18758118018 |
sales001@rokechem.com | China | 255 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 43348 | 58 |
| ChemExpress | +86-021-58950125 |
info@chemexpress.com | China | 557 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 |
sales03@chemcn.cn | China | 951 | 58 |
| Shanghai Affida new material science and technology center | +undefined15081010295 |
2691956269@qq.com | China | 359 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
Apixaban 관련 검색:
Voriconazole
Apixaban IMpurity(BMS-591329-01)
5-chloro-N-(4-(5-morpholino-6-oxo-3,6-dihydropyridin-1(2H)-yl)phenyl)pentanamide
Apixaban IMpurity(
1,3-bis(4-bromophenyl)propan-2-one
3-Chloro-1-(4-nitrophenyl)-5,6-dihydropyridin-2(1H)-one
3-Morpholino-1-(4-(2-oxopiperidin-1-yl)phenyl)-5,6-dihydropyridin-2(1H)-one
Apixaban IMpurity 6
1-(4-Methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylic acid ethyl ester
Rivaroxaban
Bortezomib
Edoxaban (tosylate Monohydrate)
(+)-JQ1
MK-2206 2HCl
Dabigatran
Ethyl (2Z)-chloro[(4-methoxyphenyl)hydrazono]ethanoate
1-(4-Methoxyphenyl)-6-(4-(2-Methyl-6-oxopiperidin-1-yl)phenyl)-7-oxo-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxaMide
Apixaban IMpurity 5 (BMS-591329-01)