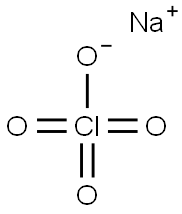과염소산나트륨 C화학적 특성, 용도, 생산
화학적 성질
Sodium perchlorate, NaCl04, is a flammable, white crystalline deliquescent solid that melts at 482 °C(899.6 °F). Soluble in water and alcohol, it is used as an analytical reagent. Sodium perchlorate is explosive in nature when in contact with concentrated sulfuric acid and finds use in explosives and jet fuels.
용도
Sodium Perchlorate is an eluent component in the analysis of samples using liquid chromatography or high performance liquid chromatography. A chaotropic salt or a chaotropic agent. A useful raw material for batteries, plastics and in rubber products.
정의
ChEBI: An inorganic sodium salt comprising equal numbers of sodium and perchlorate ions.
일반 설명
White crystalline solid. Noncombustible but will accelerate the burning of combustible materials. If large quantities are involved in a fire or the combustible material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion. Used in chemical analysis and in explosives.
공기와 물의 반응
Water soluble.
반응 프로필
Sodium perchlorate is a strong oxidizing agent. Explosively decomposed at over 130°C [Merck 11th ed. 1989]. A mixture with powdered magnesium is a friction-sensitive mixture [Safety Eng. Reports 1947]. A mixture with ammonium nitrate is used as an explosive [Mellor 2 Supp. 1:608 1956]. A 1:1 mixture of the perchlorate and hydrazine exploded upon grinding, [Chem. Abs., 1969, 70, 53524].
위험도
Dangerous fire and explosion risk in contact
with organic materials and sulfuric acid.
건강위험
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
화재위험
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. A powerful oxidizer. Forms explosive mixture with acetone, 1,3-butylene glycol, 2,3butylene glycol, CaH2, charcoal, daminoethane, dimethyl formamide, ethanolamine, ethylene glycol, formamide, galactose, glycerin, hydrazine, water, NH4NO3, Mg, reducing agents, SrH2, urea. When heated to decomposition it emits toxic fumes of Cland NazO. See also PERCHLORATES.
Purification Methods
Because its solubility in water is high (2.1g/mL at 15o) and it has a rather low-temperature coefficient of solubility, sodium perchlorate is usually crystallised from acetone, MeOH, water/ethanol or dioxane/water (33g dissolved
과염소산나트륨 준비 용품 및 원자재
원자재
준비 용품