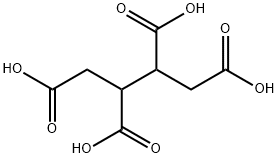
1,2,3,4-Butanetetracarboxylic acid
|
|
1,2,3,4-Butanetetracarboxylic acid 속성
- 녹는점
- 193-197 °C
- 끓는 점
- 296.47°C (rough estimate)
- 밀도
- 1.5040 (rough estimate)
- 증기압
- 0Pa at 25℃
- 굴절률
- 1.5800 (estimate)
- 저장 조건
- Sealed in dry,Room Temperature
- 용해도
- 118g/L
- 물리적 상태
- 결정성 분말
- 산도 계수 (pKa)
- pK1: 3.43;pK2: 4.58;pK3: 5.85;pK4: 7.16 (25°C)
- 색상
- 하얀색
- 수용성
- 19&C에서 >=10g/100mL
- BRN
- 1729167
- InChIKey
- GGAUUQHSCNMCAU-UHFFFAOYSA-N
- LogP
- -1.41 at 25℃
- CAS 데이터베이스
- 1703-58-8(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-36-36/37/38 | ||
| 안전지침서 | 26-37/39 | ||
| 유엔번호(UN No.) | 3261 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | EK6100000 | ||
| TSCA | Yes | ||
| HS 번호 | 29171990 | ||
| 독성 | LD50 orally in Rabbit: 1720 mg/kg | ||
| 기존화학 물질 | KE-03829 |
1,2,3,4-Butanetetracarboxylic acid C화학적 특성, 용도, 생산
화학적 성질
White leaf-shaped or needle-shaped crystals. melting point 236-237℃ for dextrorotatory-levorotatory type, 189℃ for endocyclic type. Solubility of the product in water (g/100g water): 30℃, 18.5; 50℃, 45.5; 70℃, 81.8. Soluble in ethanol. There is almost no hygroscopicity, and the purity does not decrease when left for more than two years.용도
1,2,3,4-Butanetetracarboxylic acid is used as a cross linking agent to prepare cotton cellulose in order to improve anti-prilling and flame retardant properties. It is used as a spacer in the cross-linking of titania nanoparticles to cotton. Further, it is used as a nonformaldehyde durable press finishing agent.제조 방법
1,2,3,4-butanetetracarboxylic acid is obtained by oxidation of tetrahydrophthalic anhydride.1,2,3,4-butanetetracarboxylic acid is prepared by oxidative cleavage of tetraphthalic acid or anhydride by oxidation with ozone-containing gas, followed by oxygen-containing gas, with the mixture then being heated with a peroxide, e.g. H2 O2, at 100° C. to produce the butanetetracarboxylic acid.
Process for preparing 1,2,3,4-butanetetracarboxylic acid
일반 설명
Leaflets (from water) or white powder.공기와 물의 반응
Soluble in water.건강위험
ACUTE/CHRONIC HAZARDS: When heated to decomposition 1,2,3,4-Butanetetracarboxylic acid emits acrid smoke and irritating fumes.화재위험
Flash point data for 1,2,3,4-Butanetetracarboxylic acid are not available; however, 1,2,3,4-Butanetetracarboxylic acid is probably combustible.1,2,3,4-Butanetetracarboxylic acid 준비 용품 및 원자재
원자재
구리
cis-1,2,3,6-Tetrahydrophthalic anhydride
질산
암모늄 바나딘산염
3,4-Furandiacetic acid, tetrahydro-2,5-dioxo-, 3,4-diethyl ester
Butanedioic acid, bromo-, diethyl ester
멤테트라히드로프탈산 무수물
준비 용품
1,2,3,4-Butanetetracarboxylic acid 공급 업체
글로벌( 330)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 15371 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Zhejiang ZETian Fine Chemicals Co. LTD | 18957127338 |
stella@zetchem.com | China | 2141 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 |
sales@finerchem.com | China | 2966 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Xi'an Kono chem co., Ltd., | 029-86107037 13289246953 |
info@konochemical.com | China | 2995 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
1,2,3,4-Butanetetracarboxylic acid 관련 검색:
염화 n-부틸 이소부틸브로마이드 브롬화부틸 염화 이소뷰틸 부탄 아이소부탄 테트라키스(1,2,2,6,6-펜타메틸-4-피페리딜)1,2,3,4-뷰테인테트 라카복실산 1,2,3,4-부탄에트라카복실산 혼합 1,2,2,6,6-펜타메틸-4-피페리디닐 및 트리데실 테트라에스테르
TETRAHYDROFURAN-2,3,4,5-TETRACARBOXYLIC ACID
1,2,3,4-CYCLOPENTANETETRACARBOXYLIC ACID
1,2,3,4,5,6-Cyclohexanehexacarboxylic acid
1,2,3,4-CYCLOBUTANETETRACARBOXYLIC ACID
tetrakis(2,2,6,6-tetramethyl-4-piperidyl) butane-1,2,3,4-tetracarboxylate
(2R,3S)-1,2,3,4-Butanetetracarboxylic acid,MESO-1,2,3,4-BUTANETETRACARBOXYLIC ACID
1,2,3,4-Butanetetracarboxylic acid tetramethyl ester
1,2,3,4-Butanetetracarboxylic acid-1,2:3,4-dianhydride
1,2,3,4-Butanetetracarboxylic acid dianhydride
1,2,3,4-Butanetetracarboxylic acid