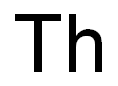THORIUM C화학적 특성, 용도, 생산
개요
Discovered in 1828 by Berzelius, thorium is a naturally occurring
radioactive metal with no stable isotopes, which is named for the
Norse god Thor. It is about as abundant as lead. Soil commonly
contains an average of about six parts of thorium per million
parts (ppm) of soil. Thorium occurs in the minerals thorite,
thorianite, orangite, and yttrocrasite, and in monazite sand.
Rocks in some underground mines may also contain thorium in
a more concentrated form. After these rocks are mined, thorium
is usually concentrated and changed into thorium dioxide or
other chemical forms. Thorium-bearing rock that has had most
of the thoriumremoved from it is called ‘depleted’ ore or tailings.
화학적 성질
Thorium is a silvery-white, soft, ductile metal which is a natural radioactive element.
물리적 성질
Thorium is a radioactive, silvery-white metal when freshly cut. It takes a month or morefor it to tarnish in air, at which point it forms a coating of black oxide. Although it is heavy,it is also a soft and malleable actinide metal. The metal has a rather low melting point, but itsoxide has a very high melting point of about 3,300°C. Thorium reacts slowly with water butreacts more vigorously with hydrochloric acid (HCl).
Thorium’s melting point is 1,750°C, its boiling point is 4,788°C, and its density is 11.79g/cm
3.
Isotopes
There are 30 radioisotopes of thorium. One isotope in particular, thorium-232,although a weak source of radiation, has such a long half-life (1.405×10
+10 years, orabout 14 billion years) that it still exists in nature and is considered stable.
Origin of Name
Thorium was named for Thor, the Scandinavian (Norse) god of “thunder.”
출처
Thorium is the 37th most abundant element found on Earth, and it makes up about0.0007% of the Earth’s crust. It is mostly found in the ores of thorite, thorianite (the oxide ofthorium), and monazite sand. It is about as abundant as lead in the Earth’s crust. As a potentialfuel for nuclear reactors, thorium has more energy potential than the entire Earth’s supply ofuranium, coal, and gas combined.
Characteristics
Thorium is chemically similar to hafnium (
72Hf ) and zirconium (
40Zr), located just above itin group 4 (IVB). Thorium-232 is found in nature in rather large quantities and goes througha complicated decay process called the thorium decay series. This series involves both alphaand beta emissions, as follows: Th-232 →Ra-228→Ac-228→Th-228→Ra-224→Rn-220→Po-216→Po-212→Pb-212→Bi-212→Ti-208→Pb-208. Thorium-232 can also be convertedinto thorium-233 or uranium-233 by bombarding it with neutrons. This results in Th-232adding a neutron to its nucleus, thus increasing its atomic weight. It then decays into uranium-233. This makes it potentially useful as an experimental new type of fissionable materialfor use in nuclear reactors designed to produce electricity.
용도
Thorium has several commercial uses. For example, thorium oxide (ThO
2) has several uses,including in the Welsbach lantern mantle that glows with a bright flame when heated by agas burner. Because of the oxide’s high melting point, it is used to make high-temperaturecrucibles, as well as glass with a high index of refraction in optical instruments. It is alsoused as a catalyst in the production of sulfuric acid (H
2SO
4), in the cracking procedures inthe petroleum industry, and in the conversion of ammonia (NH
3) into nitric acid (HNO
3).Thorium is used as a “jacket” around the core of nuclear reactors, where it becomes fissionableuranium-233 that is then used for the nuclear reaction to produce energy. Additionally,it is used in photoelectric cells and X-ray tubes and as a coating on the tungsten used to makefilaments for light bulbs. It has great potential to supplant all other nonrenewable energysources (i.e., coal, gas, and atomic energy). Thorium-232 can be converted into uranium-233,a fissionable fuel available in much greater quantities than other forms of fissionable materialsused in nuclear reactors.
생산 방법
Thorium is extracted from monazite sand concentrates for
metallurgical and other purposes by digestion with either hot,
fuming sulfuric acid or caustic soda. The resultant mass is
diluted with water that dissolves thorium, uranium, and rare
earth metals, leaving unreacted monazite, silica, rutile
(TiO2), and zircon (ZrSiO4). Neutralization of the liquor
precipitates thorium phosphate, leaving behind uranium and
most of the rare earth metals.
In 1974, U.S. domestic use of thorium was about 80 tons,
about one-half of which was employed to produce nuclear
fuels and for nuclear research. Principal nonenergy applications applications
were in the production of Welsbach incandescent
gaslight mantles, as a hardener in Th–Mg alloys, in thoriated
tungsten electrodes, and for chemical catalytic uses.
Overall, the consumption of thorium in the United States
has decreased significantly over the past several decades as
nonradioactive substances have replaced thorium in many
applications.
정의
A toxic
radioactive element of the actinoid series
that is a soft ductile silvery metal. It has
several long-lived radioisotopes found in a
variety of minerals including monazite.
Thorium is used in magnesium alloys, incandescent
gas mantles, and nuclear fuel
elements.
Symbol: Th; m.p. 1780°C; b.p. 4790°C
(approx.); r.d. 11.72 (20°C); p.n. 90;
r.a.m. 232.0381.
일반 설명
Silver to grayish radioactive metal. Twice as dense as lead. Radioactive materials emit ionizing radiation, detectable only using special instruments. Exposure to intense levels of radiation or prolonged exposure to low levels can be harmful. Film is also damaged by radiation.
공기와 물의 반응
Pyrophoric material, spontaneously ignites in air.
반응 프로필
THORIUM when heated with chlorine (or sulfur), reacts vigorously with incandescence [Mellor 7:208 1946-47]. When thorium is heated with phosphorus, they unite with incandescence [Svenska Akad. 1829 p.1].
위험도
Flammable and explosive in powder form.
Dusts of thorium have very low ignition points and
may ignite at room temperature. Radioactive decay
isotopes are dangerous when ingested.
건강위험
Radiation presents minimal risk to transport workers, emergency response personnel and the public during transportation accidents. Packaging durability increases as potential hazard of radioactive content increases. Undamaged packages are safe. Contents of damaged packages may cause higher external radiation exposure, or both external and internal radiation exposure if contents are released. Low radiation hazard when material is inside container. If material is released from package or bulk container, hazard will vary from low to moderate. Level of hazard will depend on the type and amount of radioactivity, the kind of material it is in, and/or the surfaces it is on. Some material may be released from packages during accidents of moderate severity but risks to people are not great. Released radioactive materials or contaminated objects usually will be visible if packaging fails. Some exclusive use shipments of bulk and packaged materials will not have "RADIOACTIVE" labels. Placards, markings and shipping papers provide identification. Some packages may have a "RADIOACTIVE" label and a second hazard label. The second hazard is usually greater than the radiation hazard; so follow this GUIDE as well as the response GUIDE for the second hazard class label. Some radioactive materials cannot be detected by commonly available instruments. Runoff from control of cargo fire may cause low-level pollution.
Safety Profile
Suspected carcinogen.
Taken internally as Th02, it has proven to
be carcinogenic due to its radioactivity. On
an acute basis it has caused dermatitis.
Flammable in the form of dust when
exposed to heat or flame, or by chemical
reaction with oxidizers. The powder may
ignite spontaneously in air. Potentially
hazardous reactions with chlorine, fluorine,
bromine, oxygen, phosphorus, silver, sulfur,
air, nitryl fluoride, peroxyformic acid.
잠재적 노출
Metallic thorium is used in nuclear reactors to produce nuclear fuel; in the manufacture of incandescent mantles; as an alloying material, especially with some of the lighter metals, for example, magnesium as a reducing agent in metallurgy; for filament coatings in incandescent lamps and vacuum tubes; as a catalyst in organic synthesis; in ceramics; and in welding electrodes. Exposure may occur during production and use of thorium-containing materials, in the casting and machining of alloy parts; and from the fume produced during welding with thorium electrodes. Thorium nitrate is an oxidizer. Contact with combustibles, and reducing agents will cause violent combustion or ignition.
환경귀착
Thorium’s usage may result in release of thorium compounds
to the environment through various waste streams. As noted
above, thorium is also found naturally, particularly in monazite
sand. Thorium compounds are expected to exist in the
particulate phase if released to the atmosphere based on their
low vapor pressures and may be removed from the air by wet
and dry depositions. Th and ThO2 have low mobility in soils.
In aquatic releases, adsorption is expected to be the primary
means of removal from the system.
운송 방법
UN2975 Thorium metal, pyrophoric, Hazard class: 7; Labels: 7-Radioactive material, 4.2-Spontaneously combustible material. Note: UN/NA 2975 doesn’t appear in the 49 CFR Hazmat Table.
비 호환성
The powder may ignite spontaneously in air. Heating may cause violent combustion or explosion. May explosively decompose from shock, friction, or concussion. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause violent fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, nitryl fluoride; peroxyformic acid; silver, sulfur.
폐기물 처리
Recovery and recycling is in the preferred route.
THORIUM 준비 용품 및 원자재
원자재
준비 용품