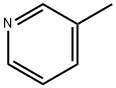
β-피콜린
|
|
β-피콜린 속성
- 녹는점
- -19 °C (lit.)
- 끓는 점
- 144 °C (lit.)
- 밀도
- 0.957 g/mL at 25 °C (lit.)
- 증기 밀도
- 3.2 (vs air)
- 증기압
- 4.4 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.504(lit.)
- 인화점
- 97 °F
- 저장 조건
- Flammables area
- 용해도
- 알코올: 혼화성(lit.)
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- 5.68(at 20℃)
- 색상
- 맑은 노란색
- 냄새
- 불쾌한 냄새
- 수소이온지수(pH)
- 10 (100g/l, H2O, 20℃)
- 폭발한계
- 1.3-8.7%(V)
- 수용성
- 녹는
- Merck
- 14,7401
- BRN
- 1366
- Dielectric constant
- 11.1
- 안정성
- 안정적인. 가연성. 흡습성. 산화제와 호환되지 않습니다.
- InChIKey
- ITQTTZVARXURQS-UHFFFAOYSA-N
- LogP
- 1.2 at 20℃
- CAS 데이터베이스
- 108-99-6(CAS DataBase Reference)
- IARC
- 3 (Vol. 122) 2019
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 10-20/21/22-34-36/37/38-22 | ||
| 안전지침서 | 16-26-36/37/39-45-36 | ||
| 유엔번호(UN No.) | UN 2313 3/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | TJ5000000 | ||
| 자연 발화 온도 | ~1000 °F | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | III | ||
| HS 번호 | 29333999 | ||
| 유해 물질 데이터 | 108-99-6(Hazardous Substances Data) | ||
| 독성 | bird - wild,LD50,oral,1gm/kg (1000mg/kg),Archives of Environmental Contamination and Toxicology. Vol. 12, Pg. 355, 1983. | ||
| 기존화학 물질 | 2003-3-2408 |
β-피콜린 C화학적 특성, 용도, 생산
화학적 성질
colourless liquid출처
3-Methylpyridine is released during the production of fossil fuels. It is formed as a byproduct of coke production (Naizer and Mashek 1974); is present in coal gasification wastewater (Giabbai et al 1985); is a contaminant of groundwater near underground coal gasification sites (Stuermer and Morris 1982); is a component of groundwater contaminated with coal-tar waste (Pereira et al 1983); and is found in shale oil wastewaters (Hawthorne and Sievers 1984; Hawthorne et al 1985). It is formed upon pyrolysis of wood (Yasuhara and Sugiura 1987) and is a constituent of cigarette (IARC 1986; Sakuma et al 1984) and marijuana (Merli et al 1981) smoke. 3-Methylpyridine is formed during the thermal degradation of nicotine in the burning of tobacco (Schmelz et al 1979). The chemical is also present in brewed coffee (Sasaki et al 1987) and black tea (Werkoff and Hubert 1975). 3-Methylpyridine has been detected along with other micropollutants in the Barcelona water supply (Rivera et al 1987). Methods for the biological treatment of wastewater high in the chemical have been developed (Roubiskova 1986). The biodegradability of 3-methylpyridine has been studied in various soils (Sims and Sommers 1985, 1986).용도
3-Picoline is used as a precursor in pharmaceuticals and agricultural industries. It acts as a precursor to 3-cyanopyridine, niacin, vitamin-B. It is an antidote for organophosphate poisoning.생산 방법
There are three major methods of 3-methylpyridine manufacturing: (1) vaporphase reaction of acetaldehyde and ammonia with formaldehyde and/or methanol in the presence of an acidic catalyst (e.g. Si02A103); (2) extraction from bone oil; (3) dry distillation of bones or coal (Hawley 1977; Parmeggiani 1983).일반 설명
Colorless liquid with a sweetish odor .공기와 물의 반응
Highly Flammable. Water soluble.반응 프로필
3-Picoline may react with oxidizing materials . Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.건강위험
HARMFUL if swallowed, inhaled or absorbed through skin. Material is extremely destructive to tissue of the mucous membranes and upper respiratory tract, eyes and skin. Inhalation may be fatal as a result of spasm, inflammation of larynx and bronchi, chemical pneumonitis and pulmonary edema. Symptoms of exposure may include burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea and vomiting.화재위험
Special Hazards of Combustion Products: Vapors may travel considerable distance to a source of ignition and flashback. Forms explosive mixtures in air. Emits toxic fumes under fire conditions.공업 용도
3-Methylpyridine can be used as a solvent, an intermediate in the dye and resin industries, in the manufacture of insecticides, as a waterproofing agent, in synthesis of pharmaceuticals, as rubber accelerators and a laboratory reagent (Hawley 1977; Windholz et al 1983). It is also used as a chemical intermediate for niacin and niacinamide (anti-pellagra vitamins) production. U.S. production in 1978 was estimated at 1.32-2.07xl07 kg (HSDB 1988).Safety Profile
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion. Flammable when exposed to heat or flame; can react vigorously with oxidizing materials. When heated to decomposition it emits toxic fumes of NOx.Synthesis
In a vapor-phase reaction over a nickel- containing catalyst in the presence of hydrogen, 2-methylglutaronitrile gives 3-methylpiperidine, which then undergoes dehydrogenation over palladium – alumina to give 3-methylpyridine: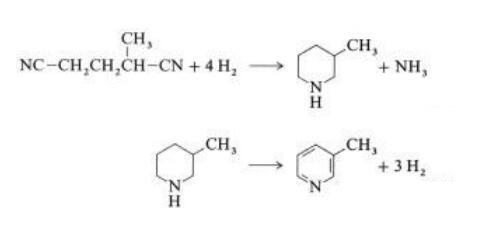
A one-step gas-phase reaction over a palladium- containing catalyst is reported to give 3-methylpyridine in 50 % yield.
잠재적 노출
(o-isomer); Suspected reprotoxic hazard, Primary irritant (w/o allergic reaction), (m-isomer): Possible risk of forming tumors, Primary irritant (w/o allergic reaction). Picolines are used as intermediates in pharmaceutical manufacture, pesticide manufacture; and in the manufacture of dyes and rubber chemicals. It is also used as a solvent.Carcinogenicity
No reliable studies in mammals to evaluate the carcinogenic potential of any of the three methylpyridines were found. None of the methylpyridines is listed as a carcinogen by IARC, NTP, OSHA, or ACGIH.신진 대사
Methylpyridines can be absorbed by inhalation, ingestion and skin contact (Parmeggiana 1983). The percentage uptake of 3-methylpyridine by rats increased with dosage; elimination occurred in 2 phases, the duration of which also was dose dependent (Zharikov and Titov 1982). Addition of a methyl group to pyridine greatly increased the rate of uptake into liver, kidney and brain of rats (Zharikov et al 1983). The position of the methyl group drastically influenced the pharmacokinetics of the methylpyridines, with 3-methylpyridine exhibiting the longest biological halflife.N-Oxidation is a minor route for 3-methylpyridine biotransformation with 6.6, 4.2, and 0.7% biotransformation of the dose, respectively, being excreted in the urine of mice, rats and guinea pigs receiving i.p. doses of the chemical (Gorrod and Damani 1980). Urinary excretion of 3-methylpyridine N-oxide was increased following pretreatment of mice with phenobarbital but 3-methylcholanthrene had no appreciable effect on N-oxide elimination (Gorrod and Damani 1979a, 1979b). The structure of 3-methylpyridine N-oxide has been verified by mass spectrometry (Cowan et al 1978).
운송 방법
UN2313 Picolines, Hazard Class: 3; Labels: 3-Flammable liquid.Purification Methods
In general, the same methods of purification that are described for 2-methylpyridine can be used. However, 3-methylpyridine often contains 4-methylpyridine and 2,6-lutidine, neither of which can be removed satisfactorily by drying and fractionation, or by using the ZnCl2 complex. Biddiscombe and Handley [J Chem Soc 1957 1954], after steam distillation as for 2-methylpyridine, treated the residue with urea to remove 2,6-lutidine, then azeotropically distilled with acetic acid (the azeotrope had b 114.5o/712mm), and recovered the base by adding excess of aqueous 30% NaOH, drying with solid NaOH and carefully fractionally distilling. The distillate is then fractionally crystallised by slow partial freezing. An alternative treatment [Reithoff et al. Ind Eng Chem (Anal Edn) 18 458 1946] is to reflux the crude base (500mL) for 20-24hours with a mixture of acetic anhydride (125g) and phthalic anhydride (125g) followed by distillation until phthalic anhydride begins to pass over. The distillate is treated with NaOH (250g in 1.5L of water) and then steam distilled. Addition of solid NaOH (250g) to this distillate (ca 2L) led to the separation of 3-methylpyridine which is removed, dried (K2CO3, then BaO) and fractionally distilled. (Subsequent fractional freezing would probably be advantageous.) The hydrochloride has m 85o, and the picrate has m 153o(from Me2CO, EtOH or H2O). [Beilstein 20 III/IV 2710, 20/5 V 506.]비 호환성
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Attacks copper and its alloys.β-피콜린 준비 용품 및 원자재
원자재
준비 용품
니코틴산
니코틴산아미드
플루아지포프-P-뷰틸
4,5-DIAMINO-6-MERCAPTOPYRIMIDINE
2-클로로-5-(트리플루오로메틸)피리딘
FMOC-2-AMINONICOTINIC ACID
6-(METHYLTHIO)PYRIMIDINE-4,5-DIAMINE
4-Hydroxynicotinic acid
Methyl 4-aminopyridine-3-carboxylate
5-Azaindole
3-Picoline-N-oxide
2,6-루티딘
5-ETHYLPYRIDINE-2-CARBOXYLIC ACID
METHYL 3-METHYL-4-PYRIDINECARBOXYLATE
4-AMINO-3-FORMYLPYRIDINE
2-클로로니코틴산
3-Picolyl chloride hydrochloride
2-Amino-5-methylpyridine
피리딘
3-Methyl-4-nitropyridine N-Oxide
β-피콜린 공급 업체
글로벌( 386)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 15371 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 |
bj1@bj-chem.com | China | 298 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| Jiangsu Zhongbang Pharmaceutical Co., Ltd. | 025-87151996 |
zbsales@chinaredsun.com | CHINA | 32 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9353 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Shaanxi Yikanglong Biotechnology Co., Ltd. | 17791478691 |
yklbiotech@163.com | CHINA | 296 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |